Ideal gasses
1/31
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Define macroscopic
The scale of objects or phenomena that are large enough to be observed with the naked eye
Define microscopic
The scale of objects and phenomena that are too small to be seen with the naked eye
Define a mole in terms of mass
The amount of substance that contains as many elementary entities as there are atoms in 0.012 kg (12 g) of carbon-12.
Define a mole in terms of number of atoms
The amount of substance that contains 6.02×1023 elementary entitie. This number is called the Avogadro constant and has symbol NA
What is the symbol for the Avogadro constant?
NA
Define Molar Mass
The mass of one mole of a substance
What symbol represents the number of moles?
n
What number represents the number of particles?
N
What equation links the number of moles and the number of particles?
N = n × NA
What are the five assumptions made in the kinetic theory of gasses?
The gas contains a very large number of atoms or molecules moving in random directions with random speeds.
The atoms or molecules of the gas occupy a negligible volume compared with the volume of the gas.
The collisions of atoms or molecules with each other and the container walls are perfectly elastic (no kinetic energy is lost).
The time of collisions between the atoms or molecules is negligible compared to the time between the collisions.
Electrostatic forces between atoms or molecules are negligible except during collisions.
What is an ideal gas?
A model of a gas including assumptions that simplify the behaviour of real gases
How does the kinetic theory of ideal gases explain gas pressure on a container?
The atoms or molecules in a gas are always moving, and when they collide with the walls of a container the atoms or molecules exert a force on the walls, which we call pressure.
What is the change in momentum in a collision with a wall?
Innitial velocity = u, so innitial momentum = mu
Velocity after collision = -u (speed doesn’t change but directions is opposite), so momenutum after collision = -mu
∆p = (-mu) - (mu) = -2mu
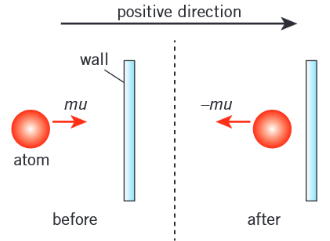
How do you derive pressure from the collision momentum?
Atoms in the gas bounce between the container walls, making frequent collisions.
According to Newton's second law, the force acting on the atom Fatom = ∆P / ∆t, where ∆p = -2mu and ∆t is the time between collisions with the wall. From Newton's third law, the atom also exerts an equal but opposite force on the wall.
A large number of atoms collide randomly with the walls of the container. If the total force they exert on the wall is F, then the pressure they exert on the wall is given by p = F / A where A is the cross-sectional area of the wall.
What is Boyle’s Law as an equation?
If mass and temperature of gas are constant:
p ∝ 1/V
pV = constant
p1/T1 = p2/T2
What is Boyle’s Law in words?
The pressure of an ideal gas is inversely proportional to its volume, provided that the mass of the gas and its temperature remain constant.
What must remain constant for Boyle’s Law and how is this achieved?
Mass and temperature of gas must remain constant
Temperature can me kept roughly the same be slowly compressing the gas
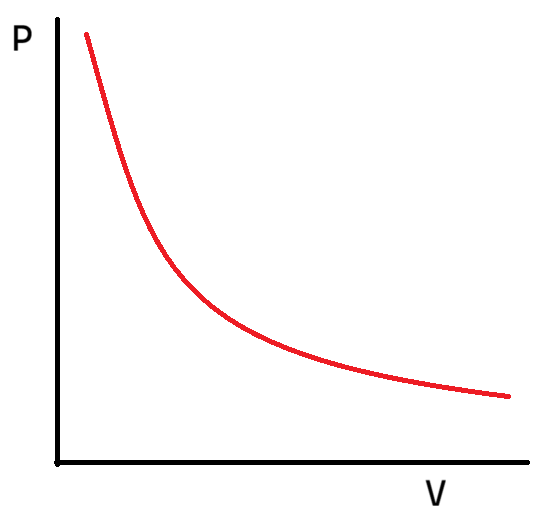
What is the graph of P against V (pressure against volume)?
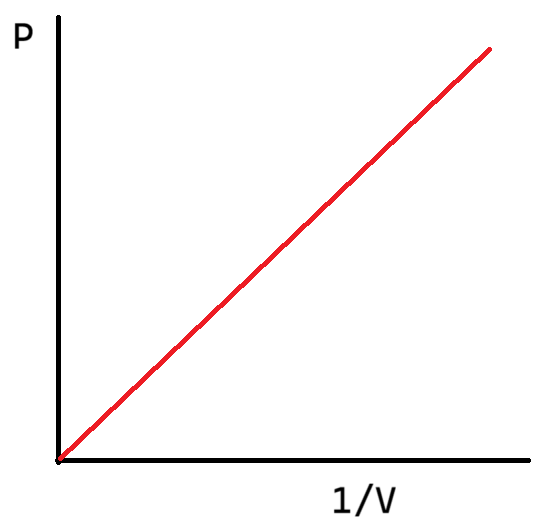
What is the graph of P against 1/V (pressure against 1/volume)?
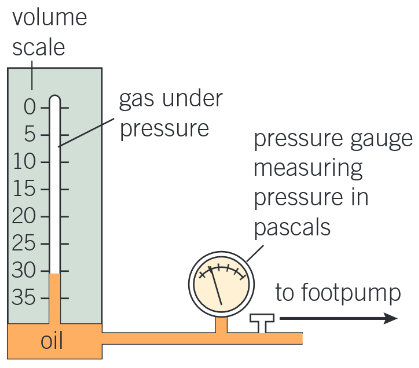
What apparatus is used to test Boyle’s Law?
What is the graph of P against T (pressure against temperature)?
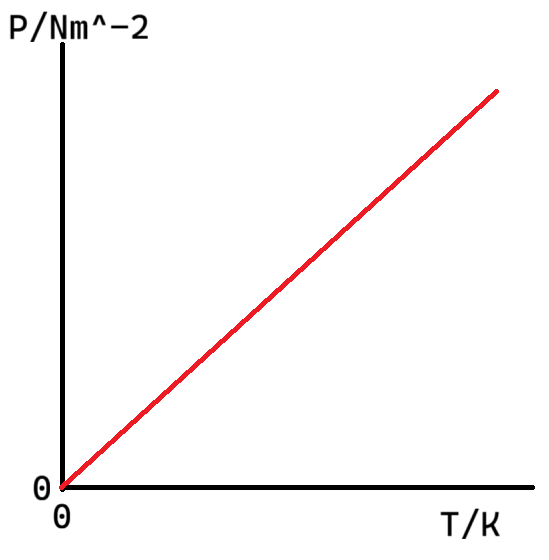
Describe the Pressure Law (relationship between P and T) in words
If the volume and mass of gas remain constant, the pressure of an ideal gas is directly proportional to its absolute (thermodynamic) temperature in kelvin.
What equation describes the relationship between P and T (the Pressure Law)?
If mass and volume of gas is constant:
p ∝ T
p/T = constant
p1V1 = p2V2
What needs to remain constant for the Pressure Law? How do we achieve this?
The volume and mass of the gass must remain constant
Add how this can be achieved
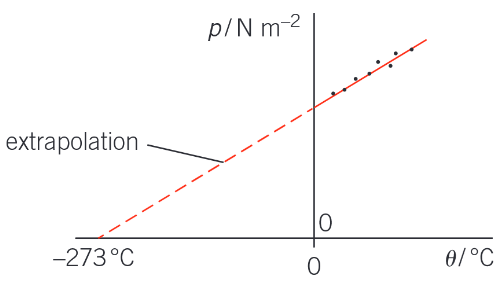
How can the experiment used to test the Pressure Law be used to find an estimate for absolute zero?
Plotting a graph of pressure against temperature θ in Celsius from the experimental results gives a line that can be extrapolated back to a point where the pressure is zero. At absolute zero the particles are not moving so the pressure of the gas must be zero.
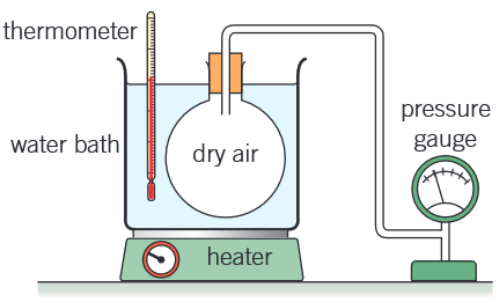
Sketch and label the apparatus used to test the Pressure Law
To be completed
What is the ideal gas equation involving n and what do the terms mean?
pV = nRT
p is pressure
V is volume of gas
n is the number of moles of gas
R is the molar gas constant
T is the temperature of the gas in kelvin
What is the ideal gas equation involving N and what do the terms mean?
pV = NkT
p is pressure
V is volume of gas
N is the number of atoms or molecules in the gas
k is Boltzmann constant (given in formula book)
T is the temperature of the gas in kelvin
What is the molar constant?
The constant in the equation of state of an ideal gas - symbol R, 8.31JK-1mol-1
What is Charle’s Law?
If the pressure and mass of a gas is constant:
V ∝ T
V/T = constant
V1/T1 = p2V2/T2
How can Boyle’s Law and the Pressure Law be combined into a single equation?
Boyle’s law → pV = constant → p1/T1 = p2/T2
Pressure law → p/T = constant → p1V1 = p2V2
Charle’s law → V ∝ T → V/T = constant → V1/T1 = V2/T2
Hence pV/T = constant
What is the internal energy of an ideal gas?
The sum of the (randomly distributed) kinetic energies of the molecules in an ideal gas.