L. 2,3,4 Alkanes, Alkenes, Alkynes, Cyclic & Aromatic Hydrocarbons
0.0(0)
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 9:27 PM on 1/24/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
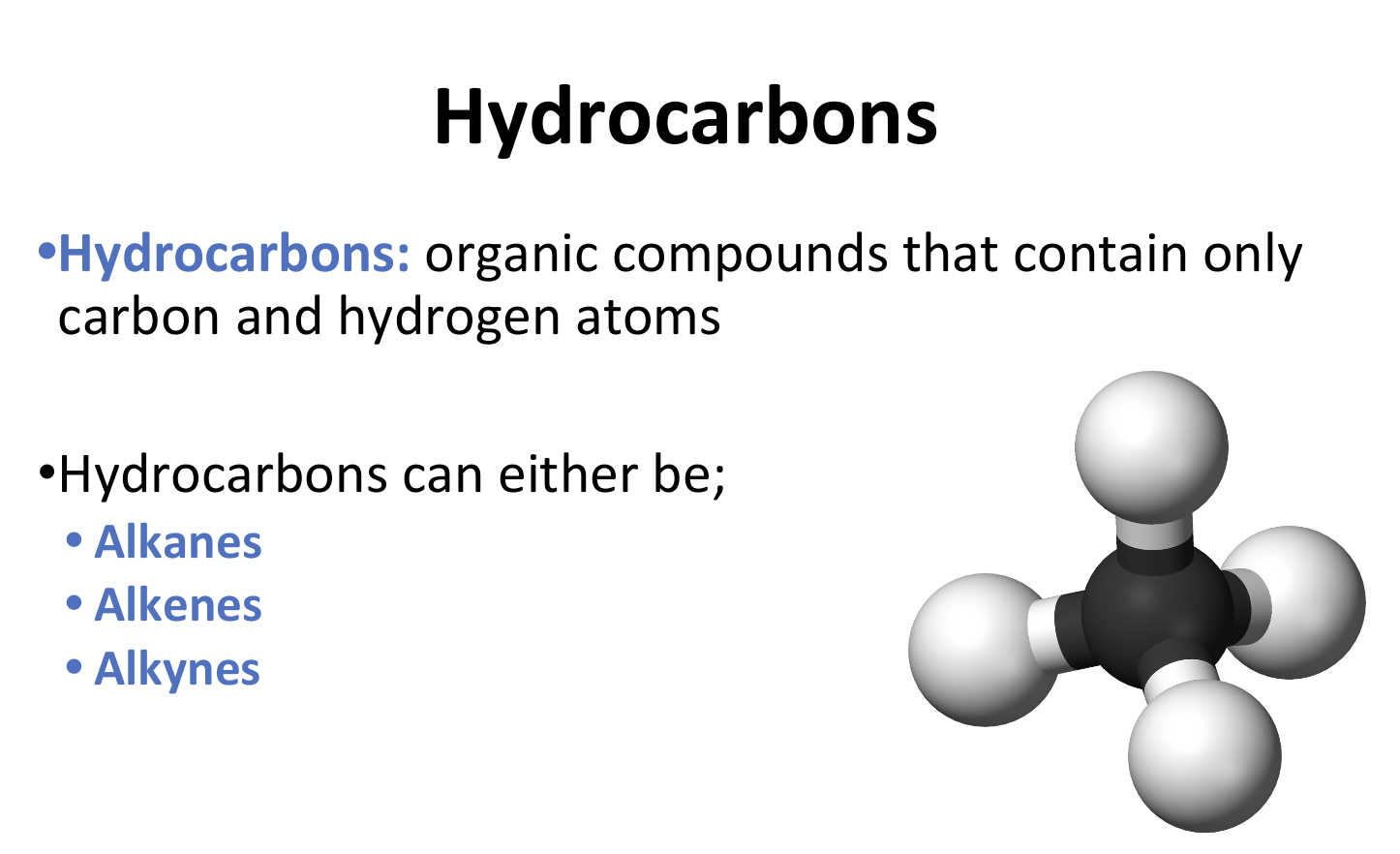
Hydrocarbons
2
New cards
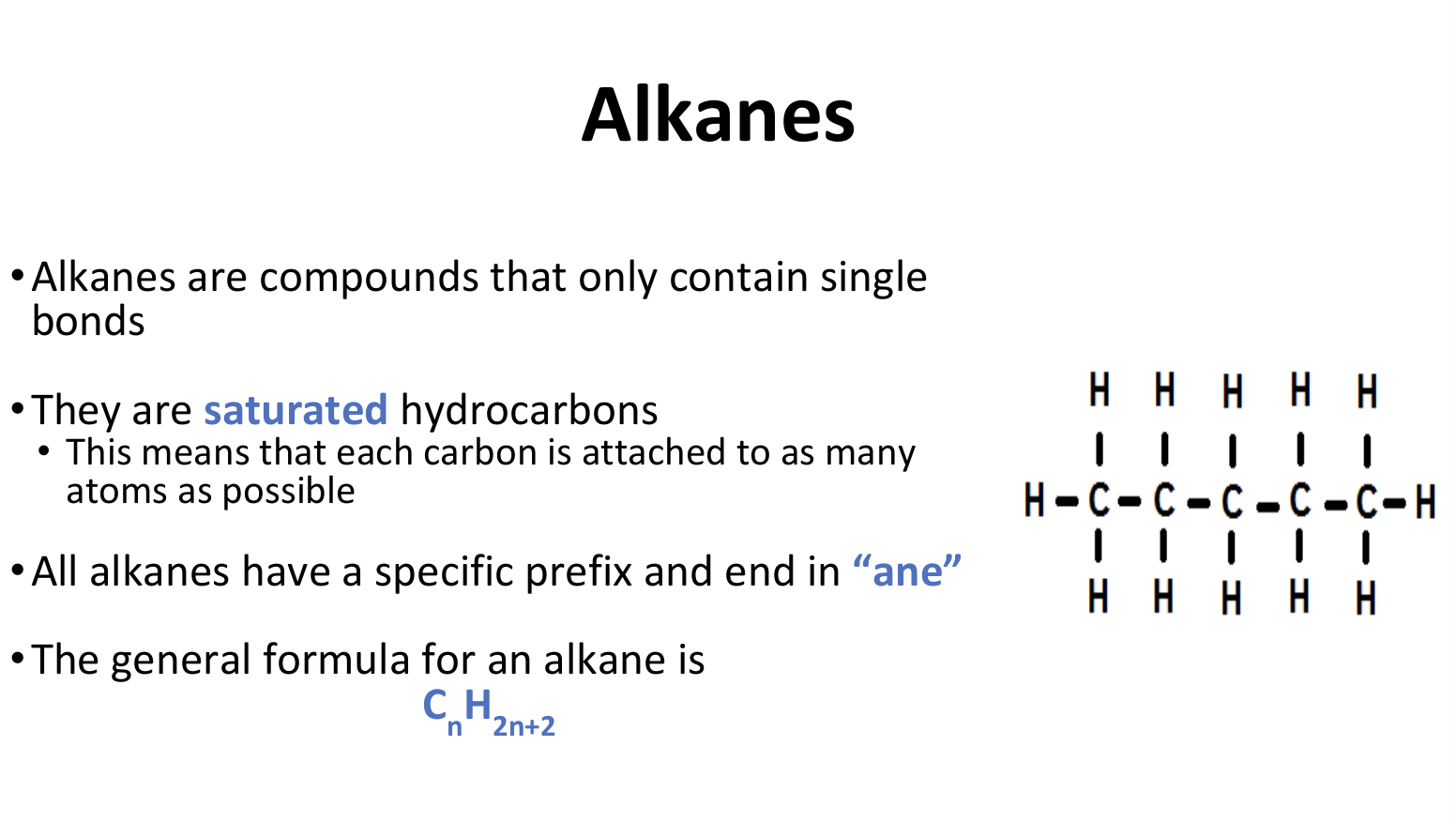
Alkanes
3
New cards
Alkane Properties

4
New cards
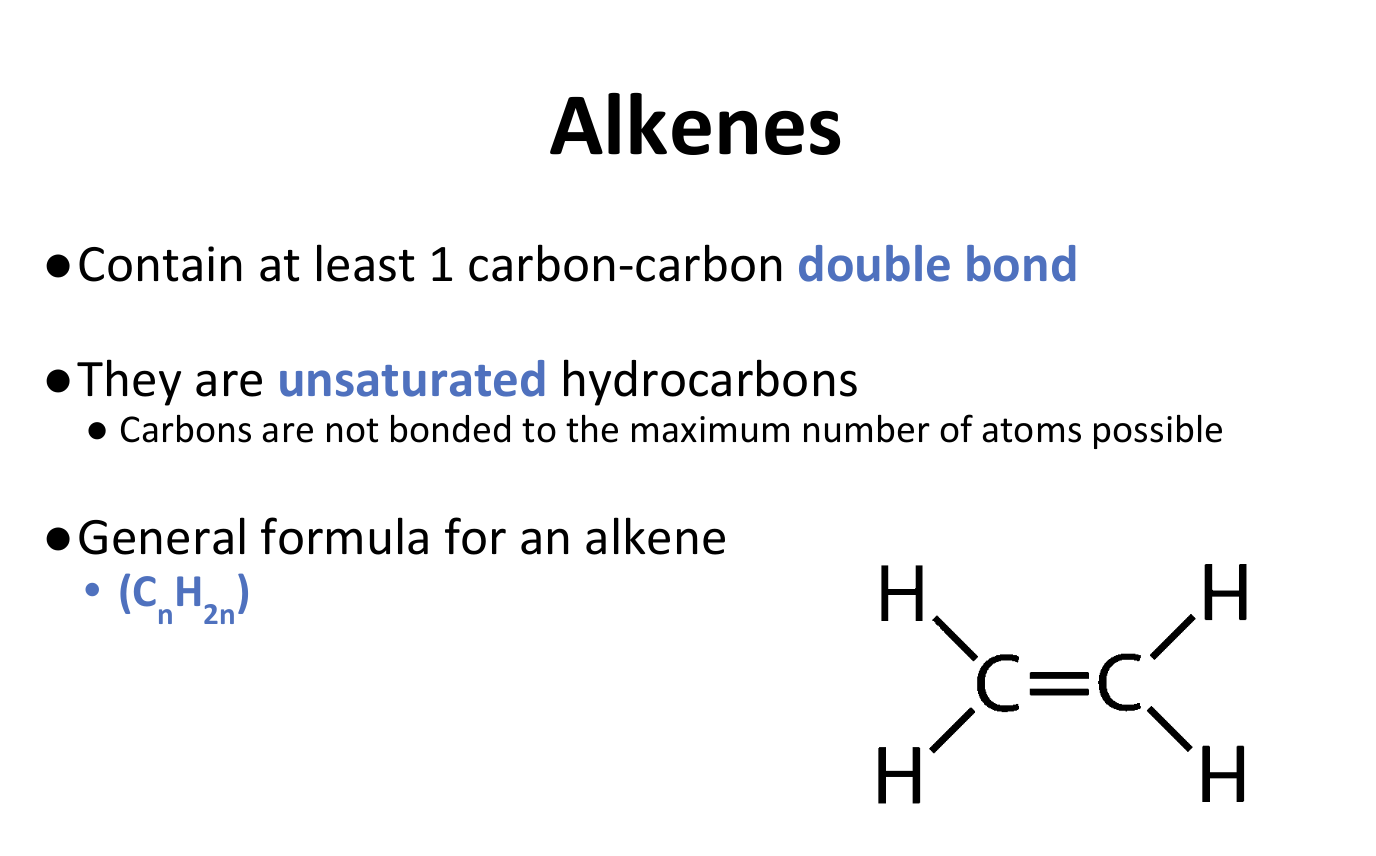
Alkenes
5
New cards
Alkene Properties

6
New cards
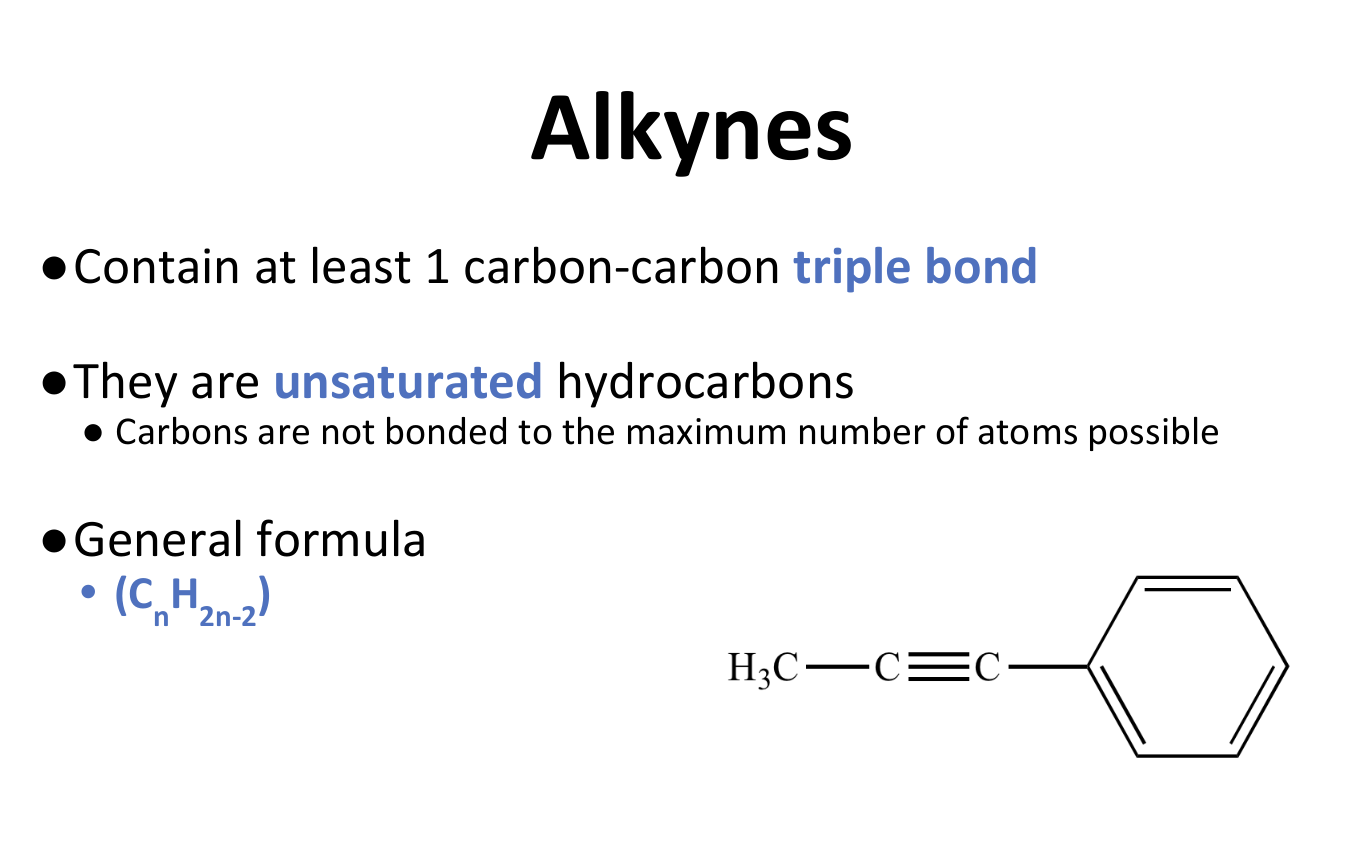
Alkynes
7
New cards
Alkyne Properties

8
New cards
Cyclic Hydrocarbons
Hydrocarbon molecules that form rings
Can be alkanes, alkenes, or alkynes
Found in many biological molecules including hormones and steroids
9
New cards
Aromatic Hydrocarbons
Molecules with a ring structure
Derive from benzene
A 6-carbon ring which has alternating single and double bonds
Referred to as resonance
10
New cards
Why does resonance occur?
Electrons in aromatic hydrocarbons are spread out (delocalized) and shared equally between the carbon atoms
Spreading makes the molecule more stable, so the electrons are less likely to react with other molecules