Immunology Exam 1
1/59
Earn XP
Description and Tags
Topics 1-3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
60 Terms
Immune System
definition
pathogenesis
Protects multicellular organisms from pathogens,
disease-causing organisms that invade a host’s tissues/cellsPathogenesis: means by which a pathogen causes disease
- A condition of an organism that impairs normal physiological function
Immune System Composition
Cells
Identify/attack pathogens, secrete anti-pathogen molecules
Tissues
Form barriers, secrete hormones & promote development of immune cells
Molecules
Dissolve in body fluids, bind and neutralize pathogens, signal cells
What are the four classes of pathogens?
bacteria
viruses
fungi
helminth (worms)
Pathogen class: Bacteria
cell type
pathogenesis
notes
complications
Type: unicellular prokaryote
Pathogenesis: colonize tissues and/or release toxins
Notes: Many foreign molecules make for easy recognition
Complications: hard to distinguish normal flora from pathogens.
Pathogen class: Virus
type
pathogenesis
notes
complications
Type: non-cellular parasite
Pathogenesis: insert DNA/RNA into host cells to create more viral particles.
Notes: Immune system must destroy both viral particles and infected host cells
Complications: many are enveloped with host cell membranes and molecules.
Pathogen class: Fungi
type
pathogenesis
notes
complications
Type: multicellular eukaryote
Pathogenesis: colonize tissues and digest them
Notes: Hyphae are resilient and infections often persist.
Complications: Physically tough hyphae and eukaryotic cell structure.
Pathogen class: Helminth (worms)
type
pathogenesis
notes
complications
Type: multicellular eukaryote
Pathogenesis: attach to vessels or gut and absorb nutrients
Notes: animal cell composition is similar to host
Complications: macroscopic structure; cannot be phagocytosed.
Leukocytes
what are they? what do they respond to? are they specific?
do they travel through the body?
what are lymphocytes?
The major cells used to combat pathogens are leukocytes (aka white blood cells) → a group of several types of cells, each with a specific role
Many leukocytes “patrol” body tissues in search of foreign entities and react to their presence with a generalized “attack” response
Lymphocytes are a subtype of leukocyte that recognize and react to specific pathogens and generate a focused (adaptive) immune response designed for total suppression/clearance of the pathogen
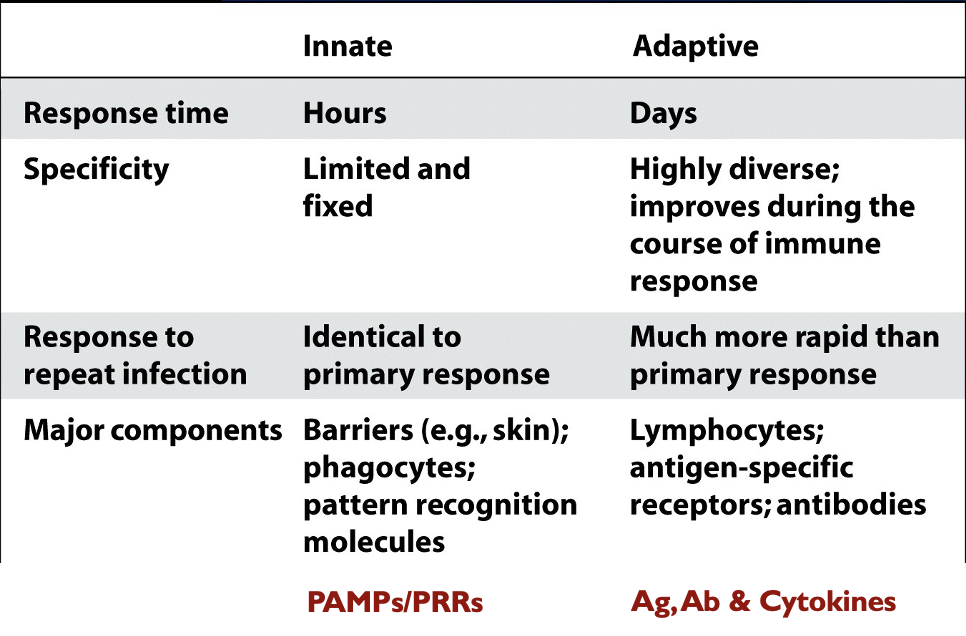
Innate Immune System
what does it involve?
when is it present in the body?
PAMPs? what recognizes them?
PRRs
Incorporates physical barriers, chemical deterrents (anti-microbial enzymes) and cells/molecules that seek and destroy non-self entities they encounter
Innate immune system components are generally always present, regardless of whether an active infection is occurring → a “perimeter defense”
Pathogen Associated Molecular Patterns (PAMPs) are non-mammalian molecules commonly found in broad groups of pathogens
Many leukocytes possess Pattern Recognition Receptors (PRRs) that bind PAMPs and trigger an attack
What are examples of PAMPs?
Lipopolysaccharide (LPS) on a bacteria
peptidoglycan
Mannose polymers → bacteria, fungi, helminths
Adaptive Immune System
Acts only in response to an infection; sometimes called acquired immunity
Does not respond immediately like innate immunity → takes several days, but response is more precise, long-lasting and completely clears pathogen
The adaptive immune system responds to antigens (Ag) → species/strain-specific molecules (usually proteins) on a particular pathogen
Two types of lymphocyte (subtype of leukocyte) respond to antigens and promote adaptive immunity
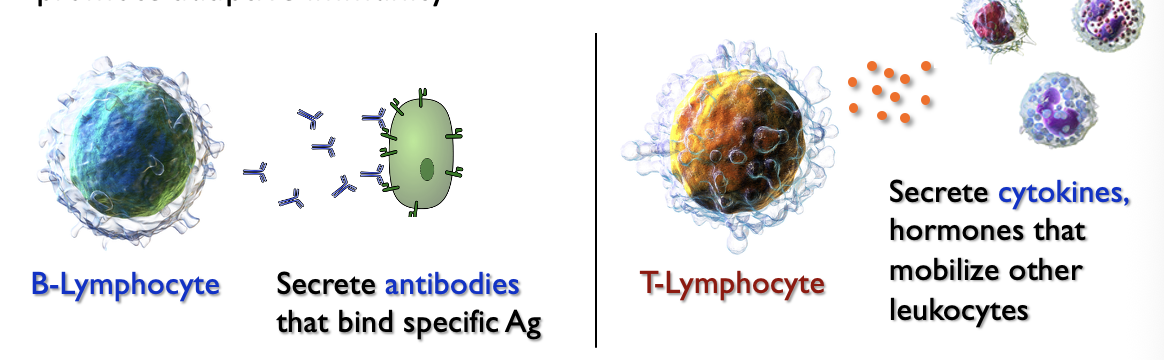
Antigens and Antibodies
Antigens (Ag) are usually macromolecules (proteins, polysaccharides, lipids, nucleic acids) found on the cell/capsid surface or secreted by the pathogen
Antigens are specific/unique to a given pathogen
Antibodies (Ab) are Y-shaped proteins that bind to specific antigens; the “tips” of the Y-shape are Ag-binding sites
This“tags” the pathogen for recognition by other leukocytes & other immune system elements
As more pathogen is encountered, more Ab is produced
Ab can “smother” a pathogen (blocks receptors/channels), while tagging it as a target
Antigen Recognition and Effector Response
Antigens are unique to a given pathogen; this is critical so ach pathogen can be identified specifically and so the effector response that follows can be tailored to best eliminate said pathogen
the adaptive immune system must also ignore normal flora
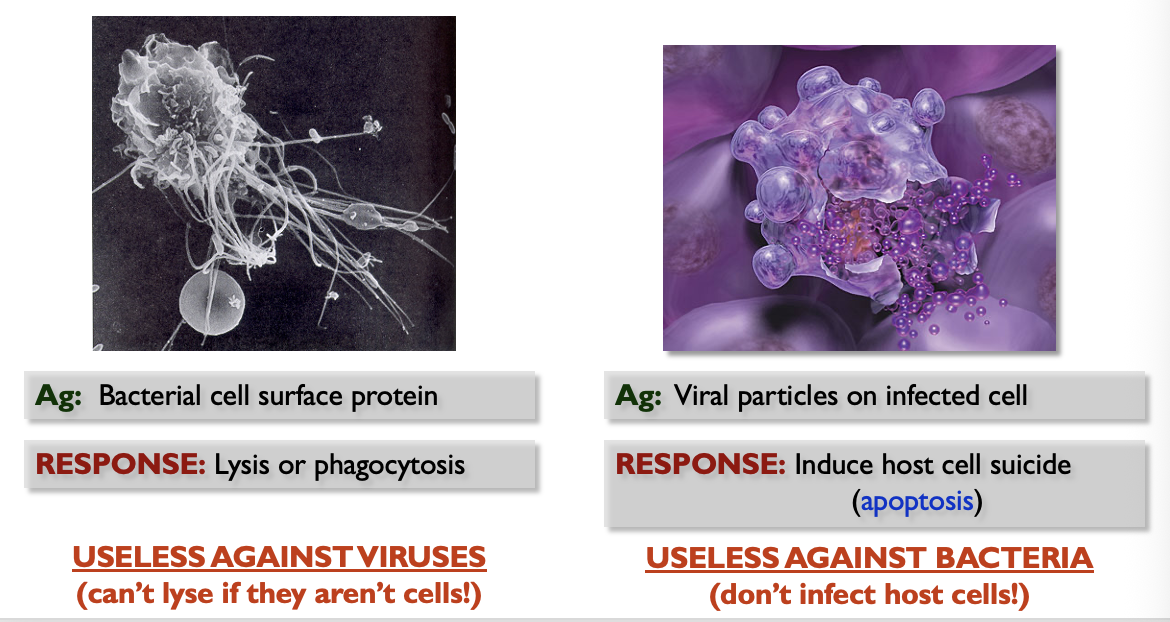
Adaptive Immunity Overview
responds to specific pathogens
has 4 main characteristics
Antigen Specificity
subtle differences between antigens are distinguishable; Ab often will not bind to an Ag with even a single aa difference
Pathogens that mutate rapidly can easily change alleles by one codon
Diversity
Billions of types of producible Ab; each binds a given Ag
contrasts strongly with generic PAMPs
Immunological Memory
memory lymphocytes are retained and respond quickly to repeat infections
Self/Non-Self Recognition
Lymphocytes that produce Ab that bind self-molecules as Ag are destroyed (this is critical so the IS doesn’t attack host)
Innate vs Adaptive Immunity
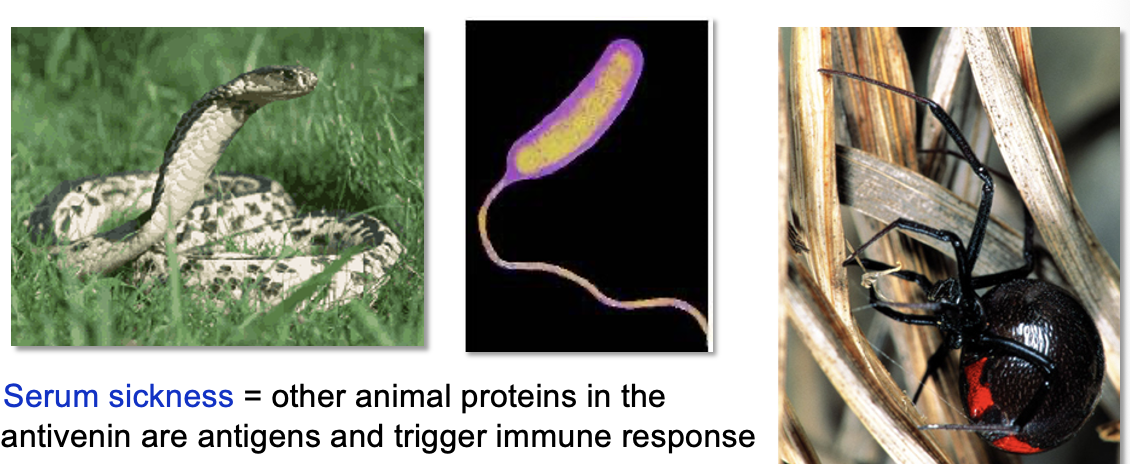
Toxins as Antigens
Antigens are often on the surface of a pathogen, but many secreted toxins (microbial or animal) are also antigens
Antivenins are made from blood serum from animals that have generated large concentrations of antibodies that bind and neutralize
Serum sickness: other animal proteins in the antivenin are antigens that trigger an immune response
tetanus, diphtheria, and cholera cause disease by releasing toxins
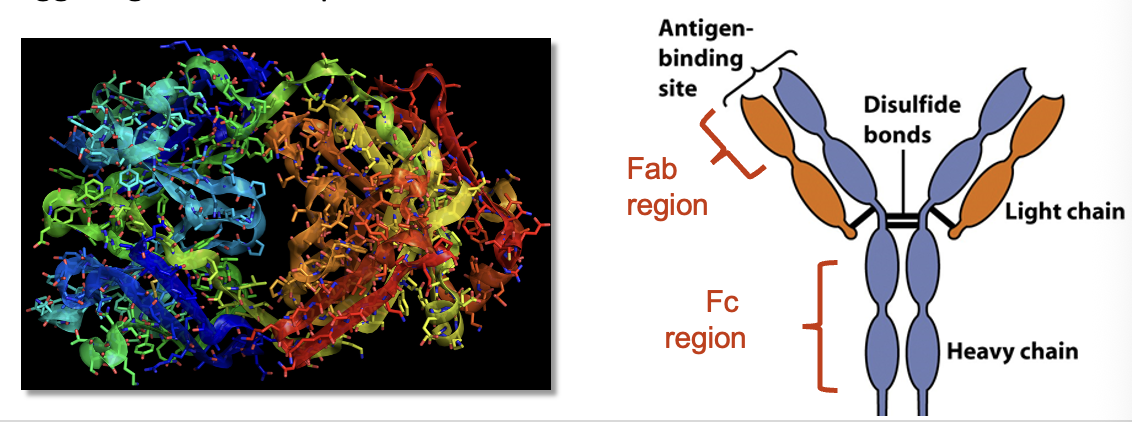
Basic Ab Structure
Antibodies are Y-shaped molecules consisting of four polypeptide chains (2 heavy, 2 light) that are bound together by disulfide bonds
Antibodies are hetero-tetramers w/ Ag Fab binding sites at the tips of the “Y”
The binding site of each Ab has a specific amino acid sequence that makes it chemically compatible with a specific Ag, like enzyme/substrate binding
Fc region of Ab “stem” can bind to receptors on leukocytes, triggering effector responses
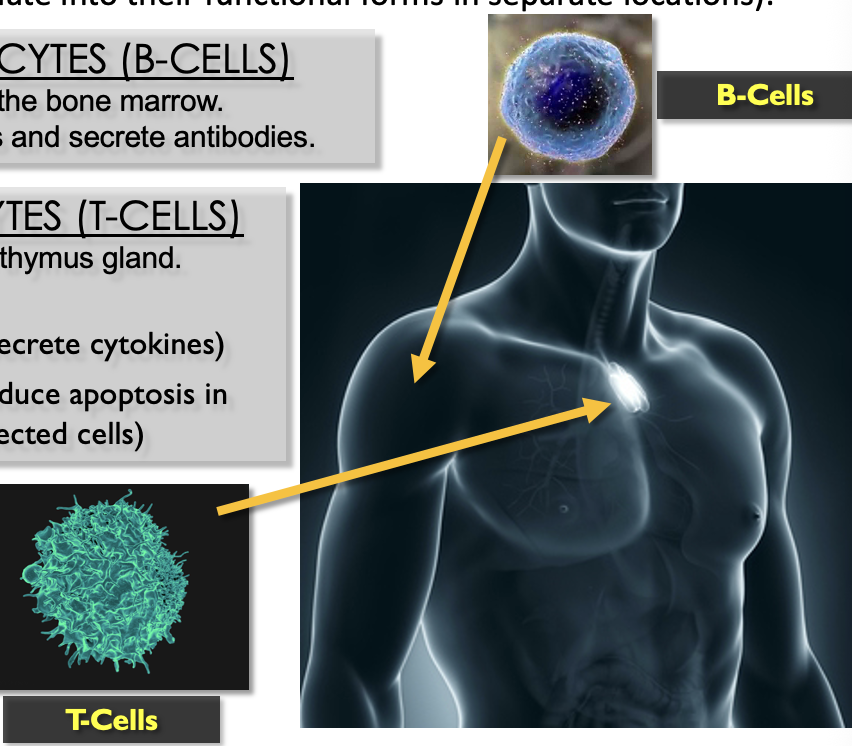
Lymphocyte Overview
two types
B-lymphocytes
mature in the bone marrow
React to antigens and secrete antibodies
T-lymphocytes
mature in the thymus gland
two types
Helper - secrete cytokines
Killer - induce apoptosis in infected cells
Clonal Selection & Expansion
through “controlled mutation” of receptor genes, each lymphocyte in the immune system creates a receptor that recognizes a different Ag
upon infection, the specific lymphocyte binds Ag and undergoes mitosis for several days, resulting in an “army” of lymphocytes against that Ag
This process is called clonal selection because all the daughter lymphocytes produced are genetically identical (recognize same Ag) as the original
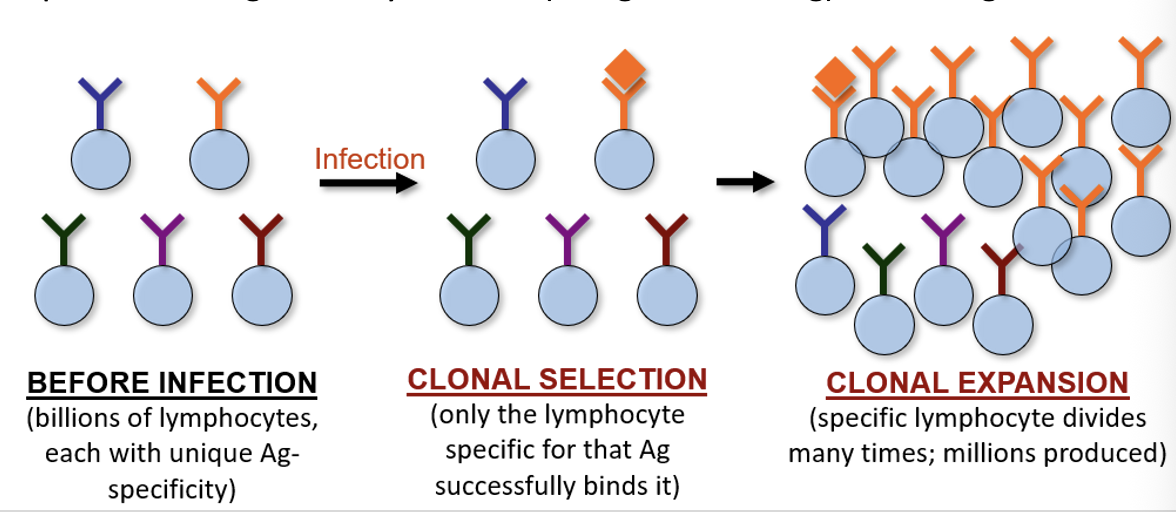
Clonal Selection in B-Cells
B-cells have an Ag-receptor called the B-Cell Receptor (BCR)
When Ag binds, the B-cell proliferates and daughter cells differentiate into plasma cells that secrete Ab into lymph and bloodstream
BCR and Ab have the same structure/gene, but BCR is membrane-bound
A smaller number of memory cells are dormant and remain after infection
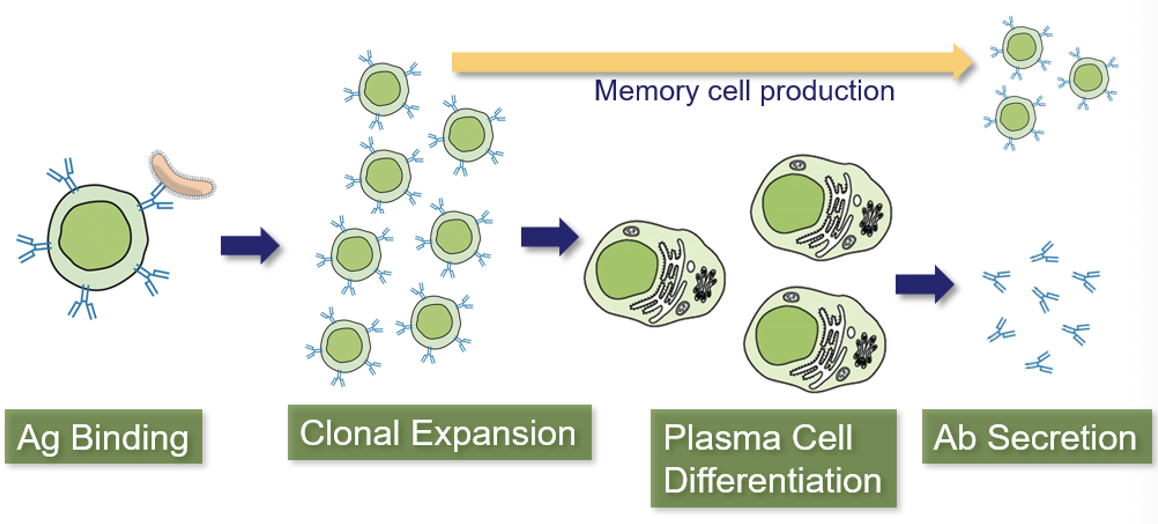
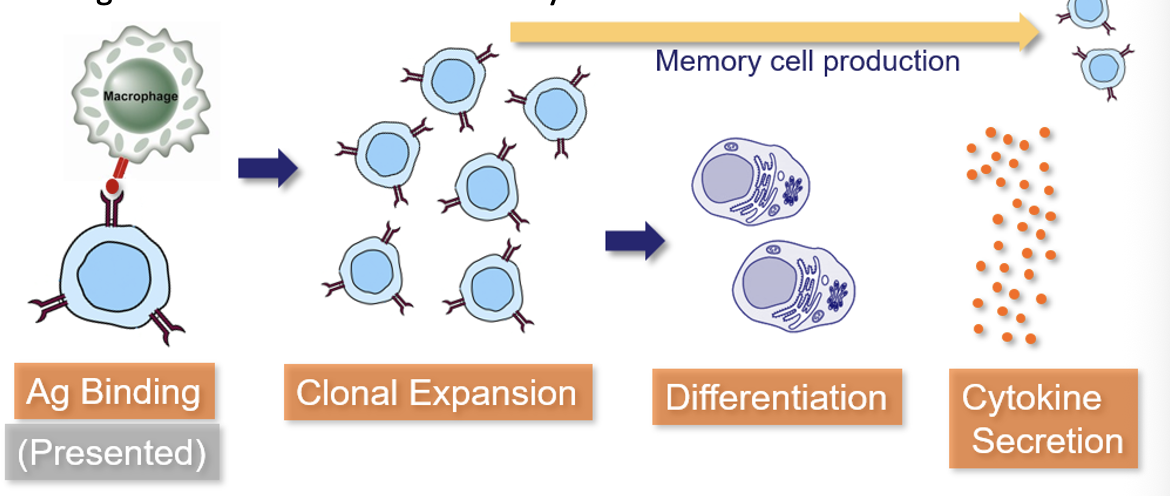
Clonal Selection in Helper T-Cells
Helper T-cells (TH) have an Ag-receptor called the T-Cell Receptor (TCR)
When Ag binds, T-cells proliferate and begin secreting cytokines
T-cells cannot bind Ag alone; it must be presented by a leukocyte that has phagocytosed the Ag and “presents” Ag particles on its surface
This Ag-Presentation is important because it prevents T-cells from reacting to Ag that hasn’t been confirmed by another cell
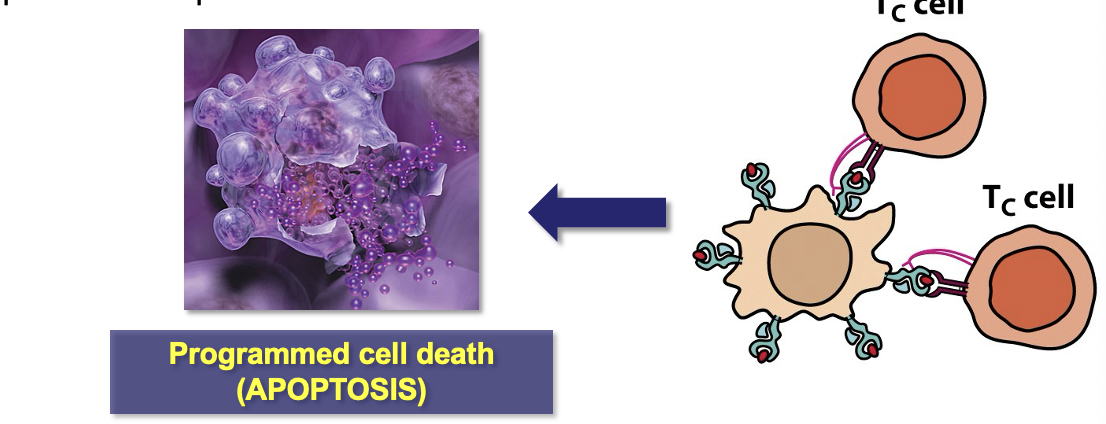
Cytotoxic T-Cells
Killer T-cells (TC) respond to the cytokines released by TH cells and convert into active cytotoxic T-lymphocytes (CTLs)
CTLs identify and destroy virally-infected host cells
Virally-infected cells display viral Ag particles on their cell surface
CTL bind to the Ag complex on infected cells and induce apoptosis to prevent the spread of virus
Humoral and Cell-Mediated Immunity
Adaptive immunity results from B-cell induced humoral immunity because it and T-cell driven cell-mediated immunity
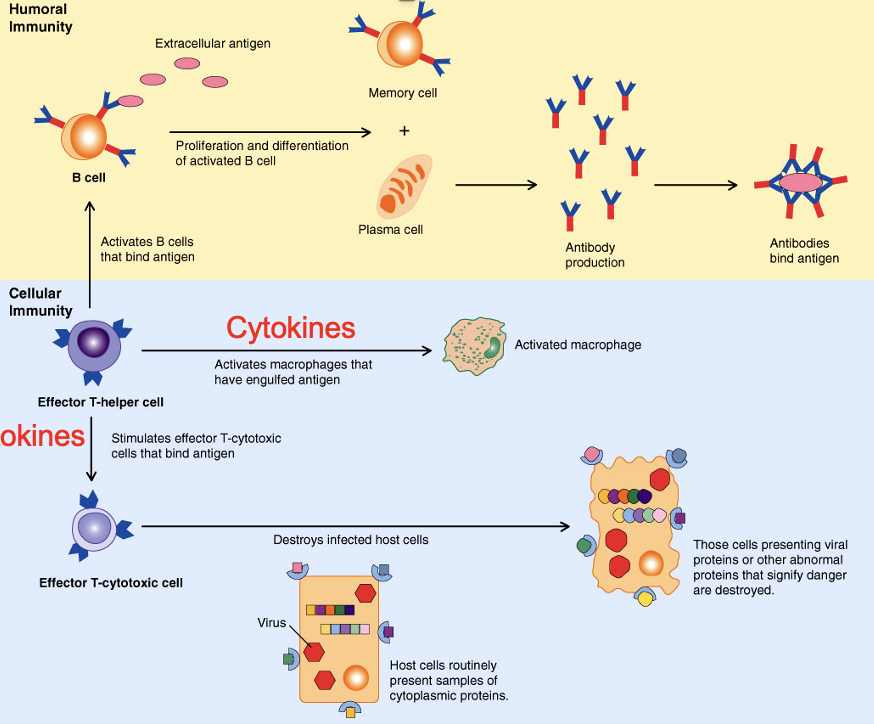
Immunological Memory
When the immune system detects an Ag, large concentrations of Ab are produced against the Ag and cytokines mobilize leukocytes
Memory cells remain dormant in the body after infection but are rapidly activated generated upon re-infection
This secondary immune response generally eliminates the pathogen before any symptoms develop; thus it’s true immunity
The immunological memory can eventually fade a memory cells undergo apoptosis after years of dormancy → Why we need booster shots
Vaccines are solutions of non-infectious Ag that induce an immune response (and memory) without causing a disease state
Some vaccinations/infections provide lifelong immunity (chickenpox) while other pathogens (cold virus, sleeping sickness) resist immune memory
Length of memory is related to the pathogen and severity of infection
Weak infections produce fewer memory cells
Some pathogens have adapted strategies to evade or “out-mutate” immunity
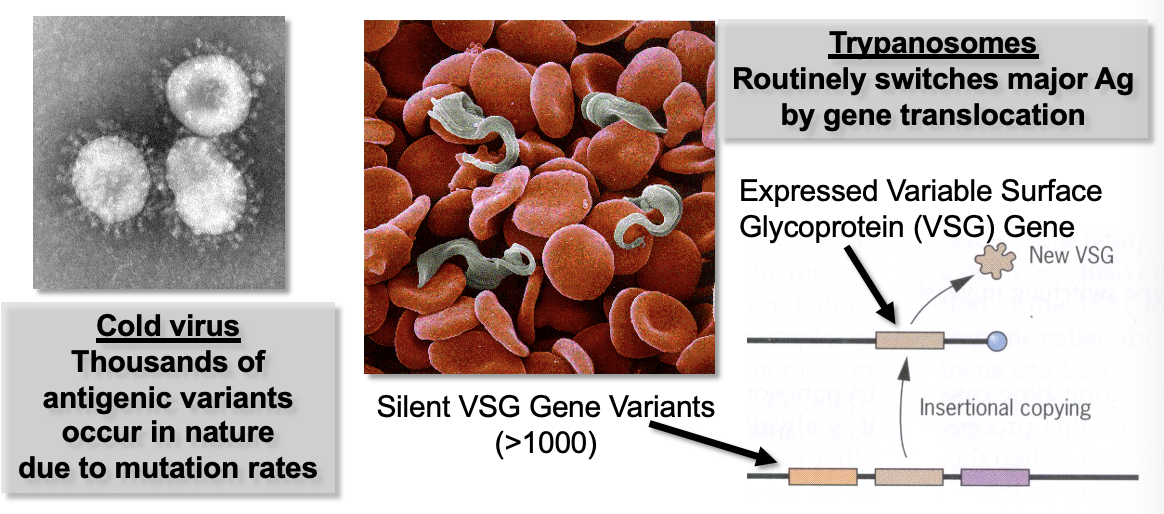
Active Immunity
the person/animal’s immune system develops their own immune cells and antibodies in response to a pathogen
the patient is challenged with Ag, generating lymphocytes & memory cells
Vaccines and infection generate active immunity
Passive Immunity
A subject is provided antibodies against a specific antigen directly (don’t produce any themselves)
Antisera (injectable Ab solutions) or antivenins are emergency treatments of infections of poisonings (e.g. tetanus) or for immunocompromised patients
Mothers provide maternal antibodies through placenta and breast milk
Passive immunity does not generate immunological memory because the host immune system is not generating the response (no memory cells)
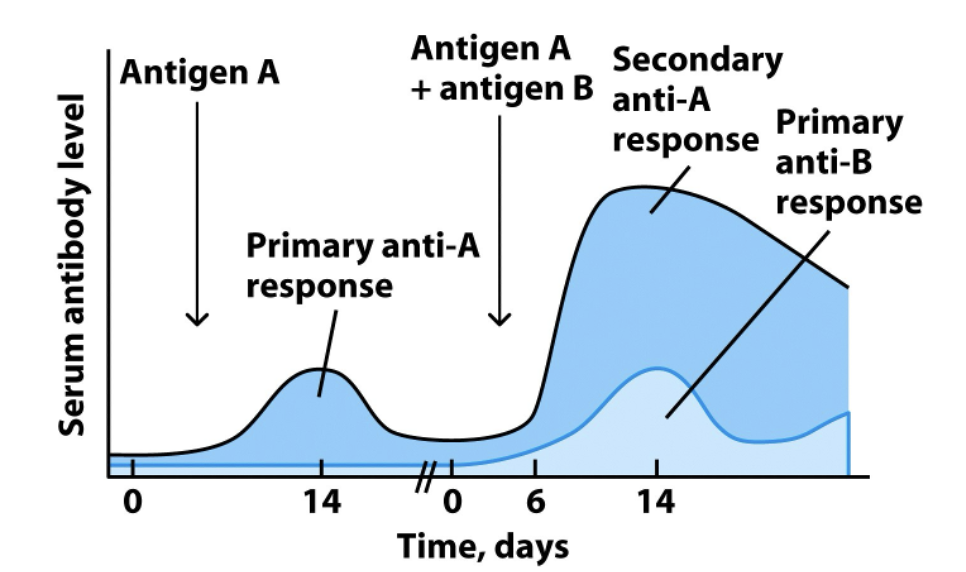
Immune Response and Memory
Due to clonal expansion and the production of memory cells, a greater amount of antibody will be produced (and with shorter lag time) on the second exposure to a given antigen
Lymphocytes produced through clonal expansion are specific to a given Ag, so they can’t help fight off a different pathogen
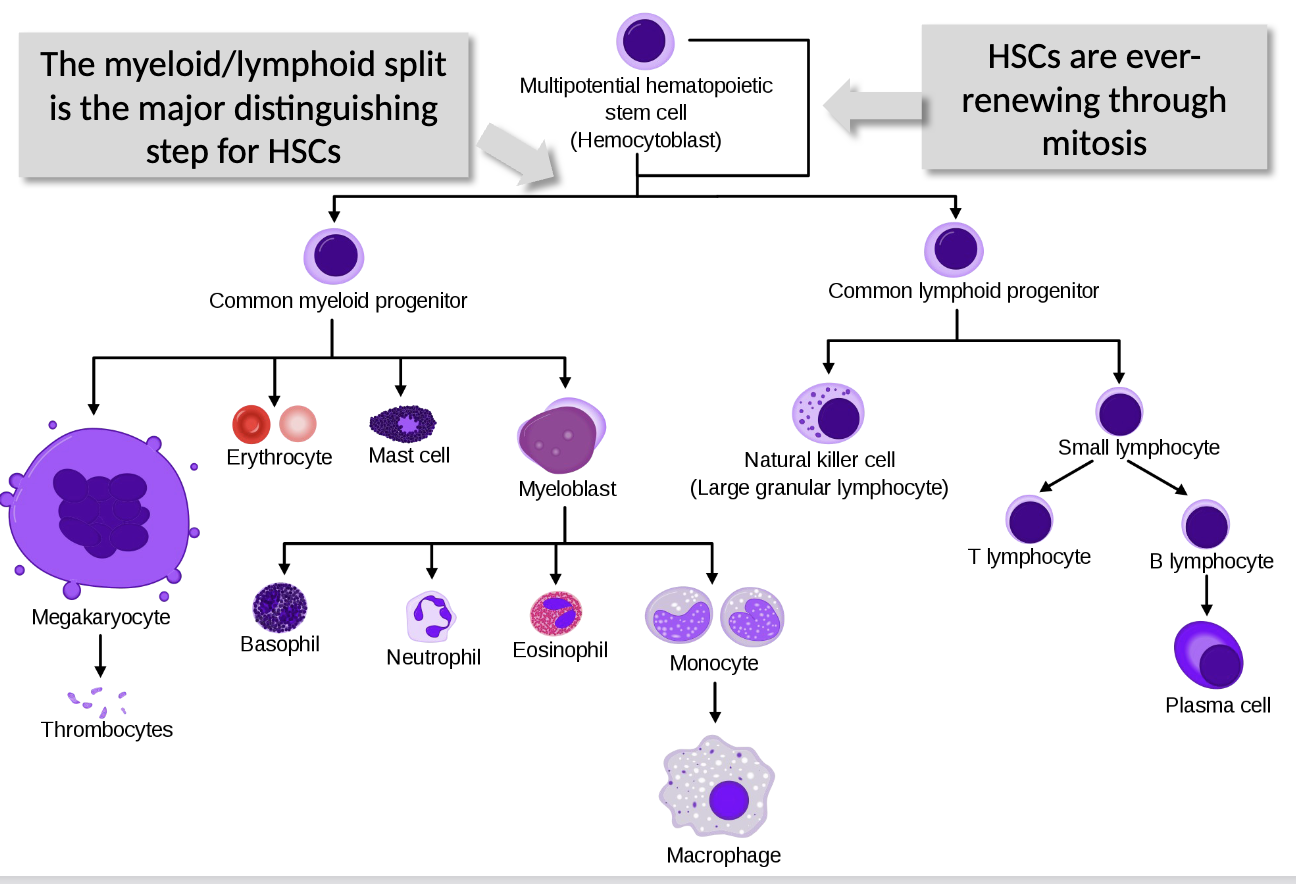
Blood cells
where are they derived from?
what is the major distinguishing step for HSCs?
do HSCs ever run out?
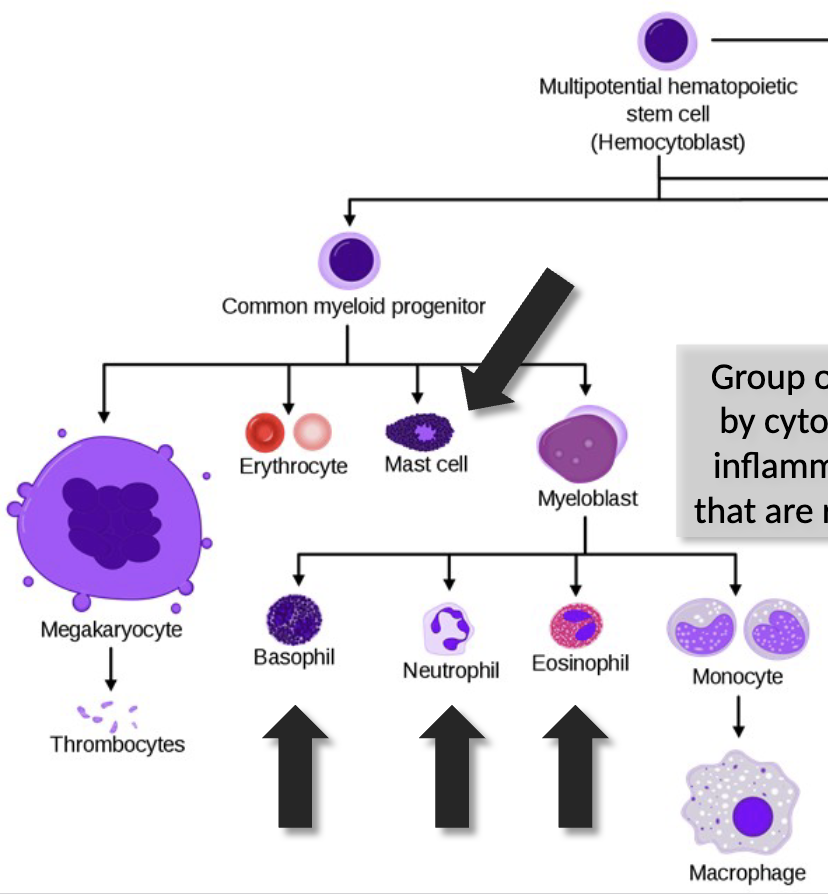
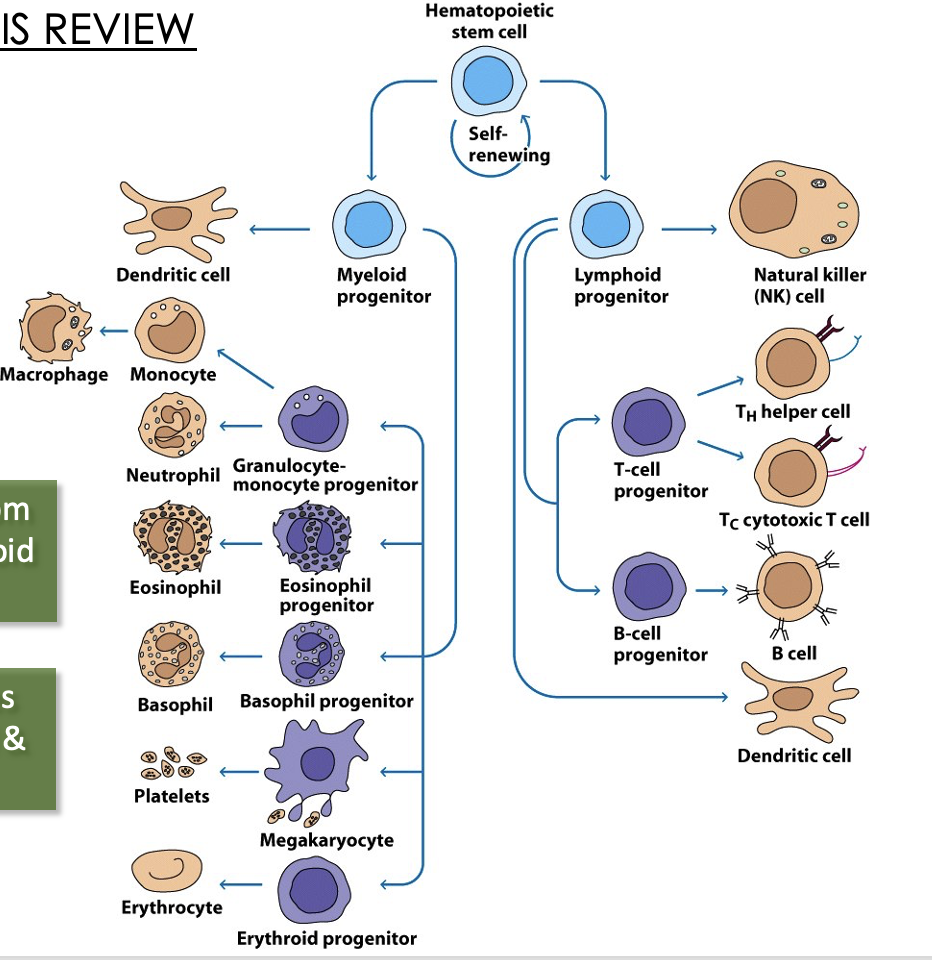
all blood cells derive from hematopoietic stem cells (HSCs) in bone marrow, and differentiate into erythrocytes and leukocytes through hematopoiesis
the myeloid/lymphoid split is the major distinguishing step for HSCs
HSCs are ever-renewing through mitosis
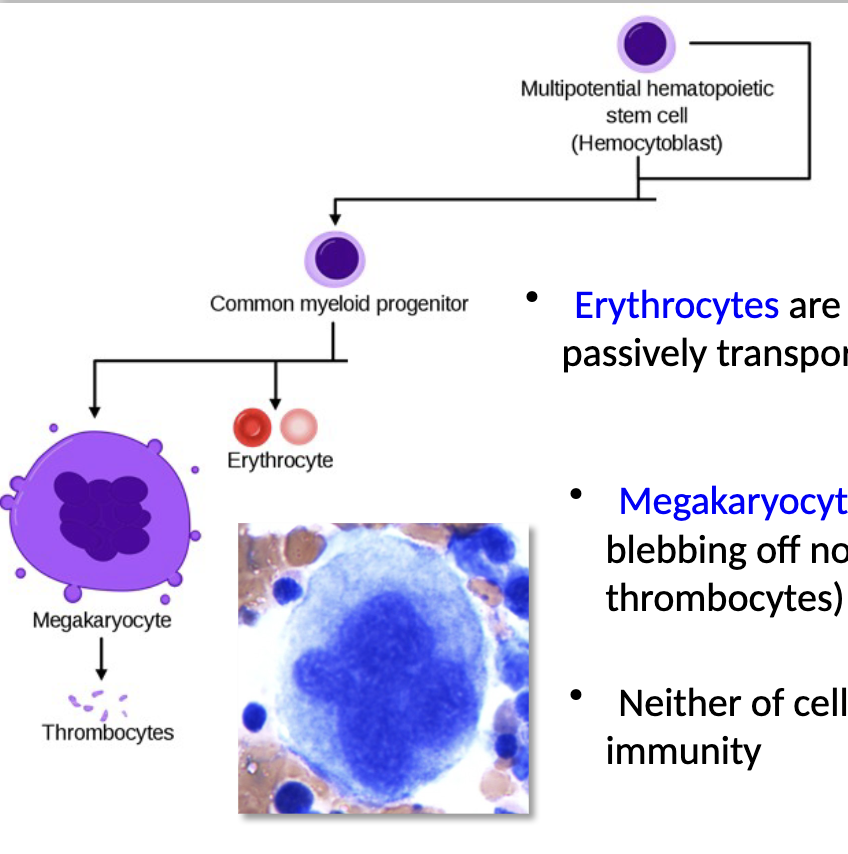
Myeloid progenitors
what do they differentiate into?
what are erythrocytes?
what are megakaryocytes?
does either cell have a direct role in immunity?
myeloid progenitors can differentiate into erythrocytes (RBCs) and megakaryocytes
erythrocytes are non-nucleated cells that passively transport O2 bound to hemoglobin
megakaryocytes respond to wounds by blebbing off non-nucleated platelets (aka thrombocytes) that form blood clots
neither of cell type has a direct role in immunity
Myeloid lineage leukocytes
what do they function as? where?
what do they respond to?
what can they detect and trigger?
function in the innate immune, as “first responders” to
infection, recognizing and responding to PAMPs with their PRRsHowever, each also has receptors that detect Ab bound to Ag and trigger aggressive effector responses
So these function in both innate & adaptive immunity
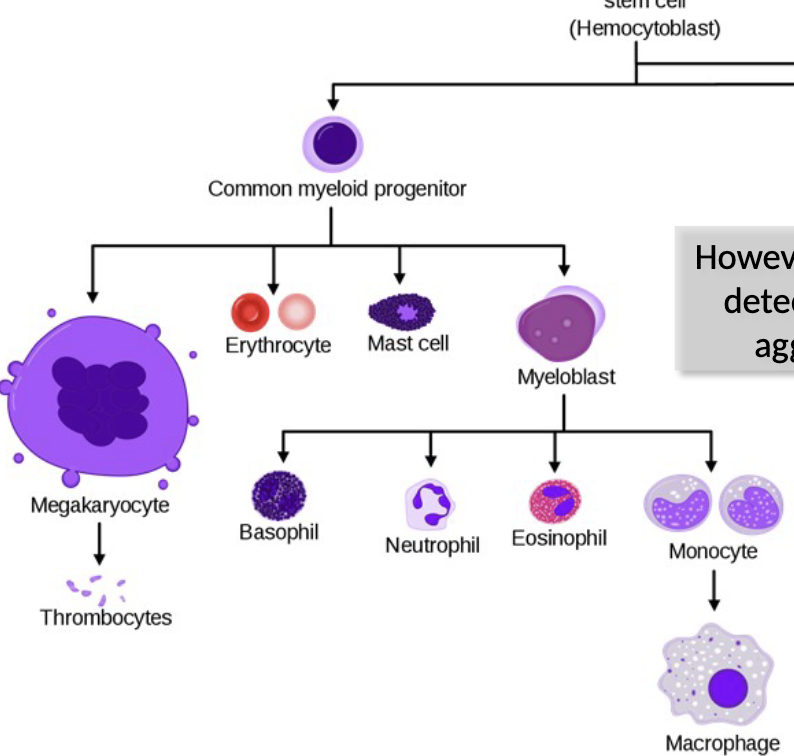
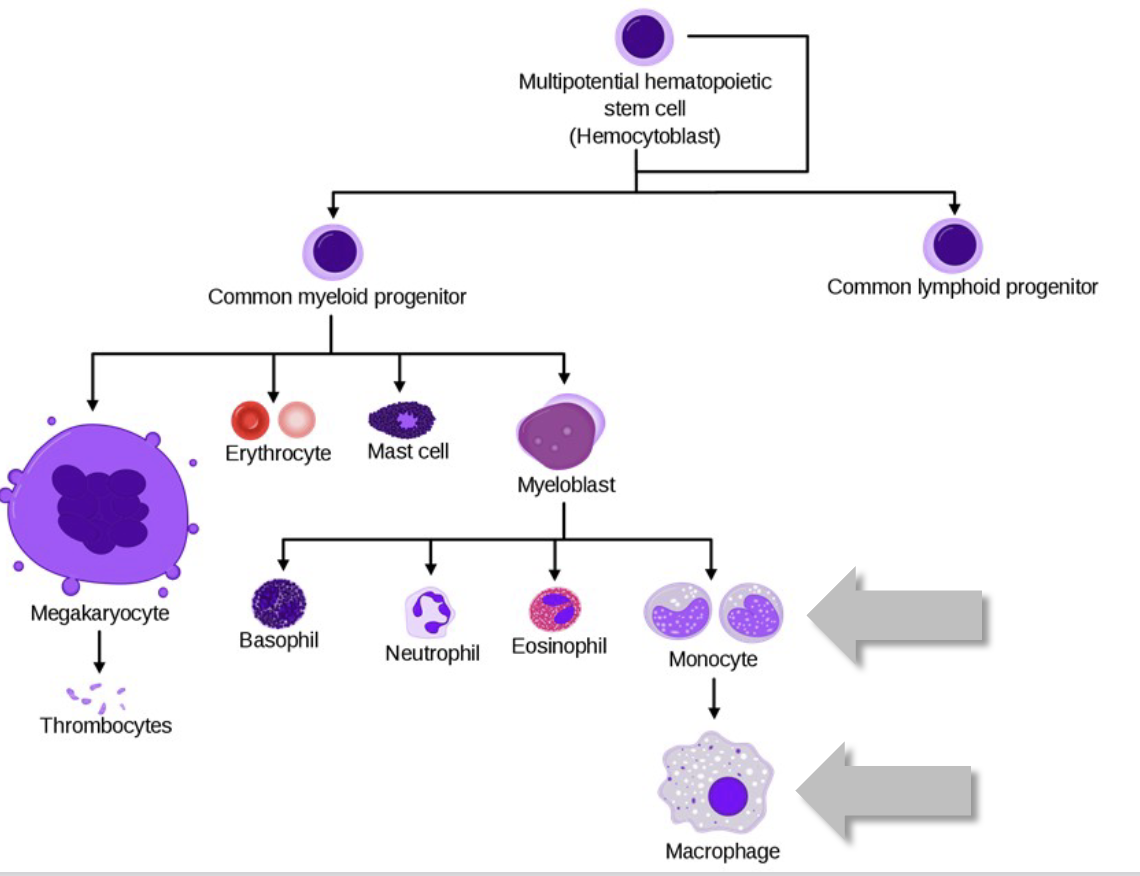
Mononuclear Phagocytes
how do these cells begin? what do they become?
what happens when they convert?
morphology?
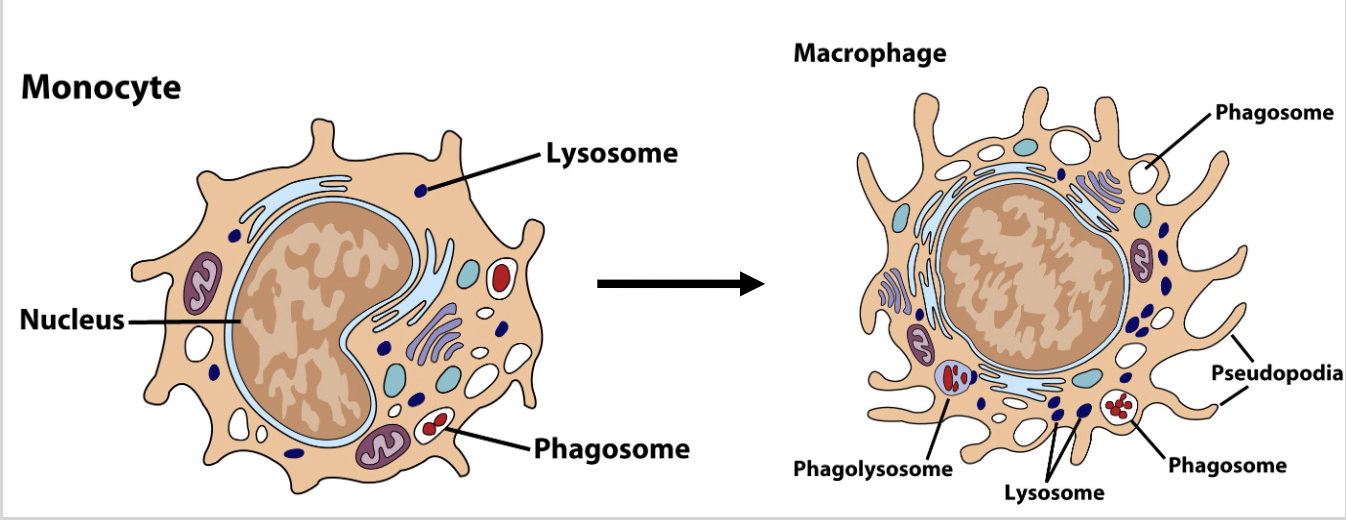
these cells begin as monocytes, circulating in blood for ~8 hours, then differentiating into amoeboid macrophages that “crawl” through tissues
When monocytes convert to macrophages, they become 5-10X bigger, gain more organelles, longer pseudopods and increased phagocytic capabilities
Macrophages can be immobile/fixed in a particular tissue or “wandering”
Morphology = mononuclear with lots of pseudopods; 5-10% of blood cells
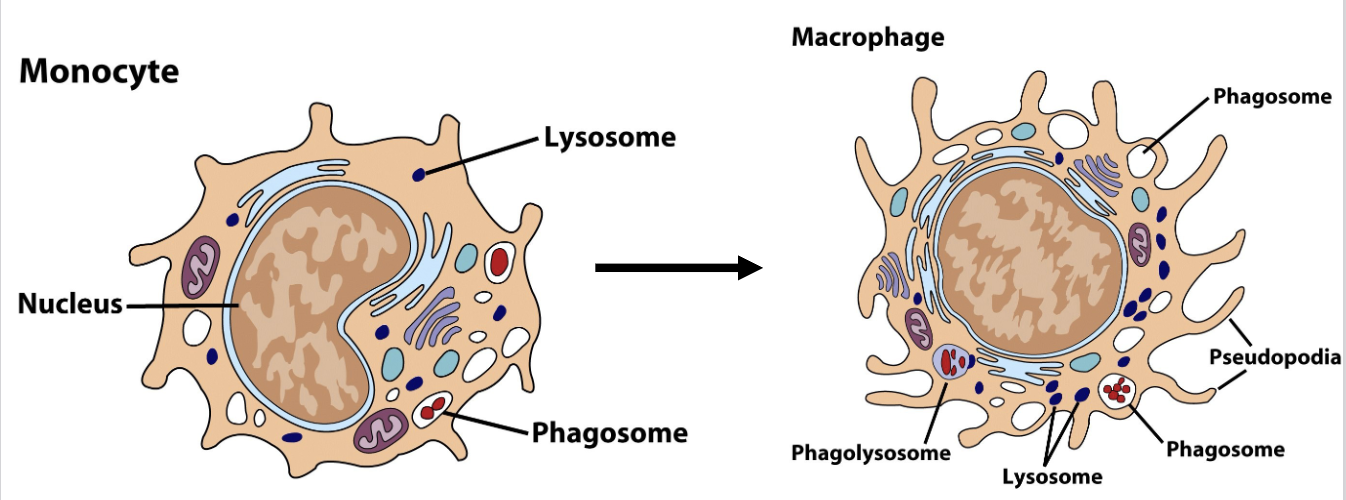
What are the differences in morphology between monocytes and macrophages?
Monocytes
bean-shaped nucleus
small pseudopods
a few lysosomes
phagosomes
Macrophage
round nucleus
aggressive, large pseudopods
many lysosomes
larger? phagosomes
phagolysosome
Macrophages
what is primary role?
what triggers phagocytosis?
Fc receptors?
how do cytokines effect activity?
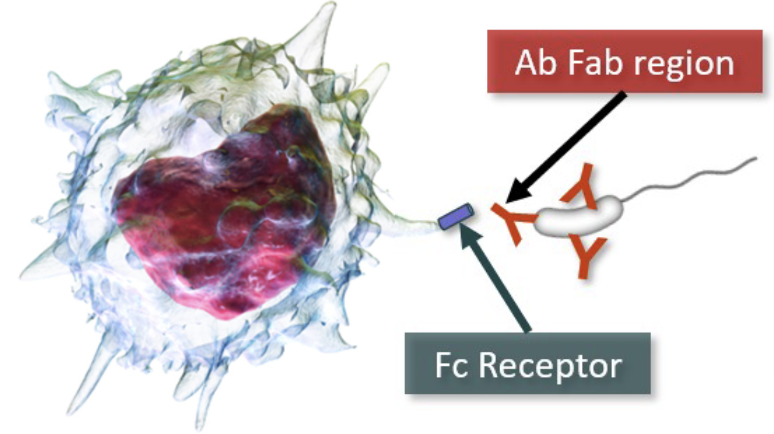
primary role is phagocytosis of pathogens, triggered by contact of pathogen with MØ pseudopods
MØ PRRs can bind to PAMPs, triggering phagocytosis as part of innate immune system response
MØ also have Fc receptors that bind Ab bound to Ag and induce phagocytosis more effectively than PRRs
Cytokines greatly increase MØ activity, aggressiveness, and pseudopod action.
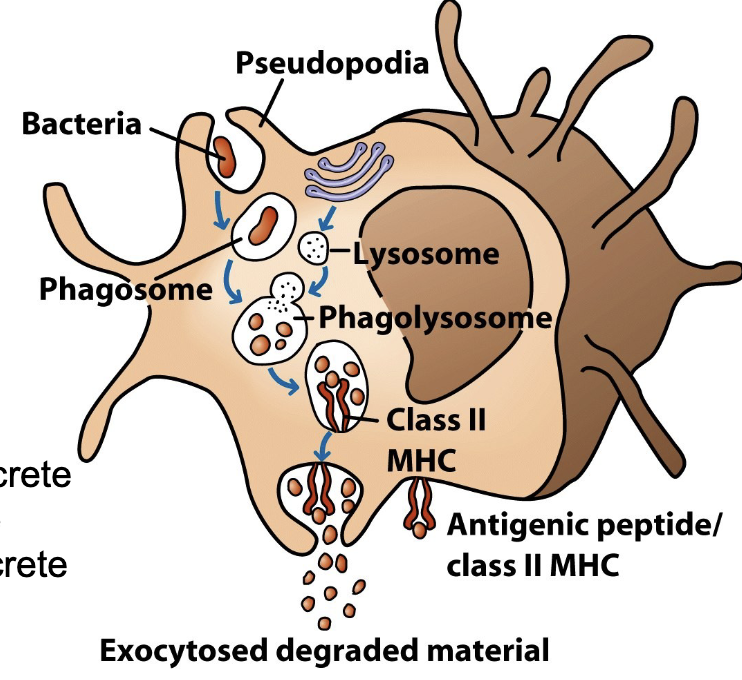
Macrophages and Antigen Presentation
When MØ ingest pathogens, they fuse the phagosome/endosome with a lysosome to digest pathogen molecules
Remaining peptide fragments are bound into a Major Histocompatibility Complex (MHC) receptor and “displayed” to T-cells to initiate adaptive immunity
MØ displaying MHC/Ag are called Ag-Presenting Cells or APCs
APC MØs become more activated after engulfing pathogen and/or in response to cytokines
Increase phagocytic activity, secrete inflammatory molecules, produce antimicrobial substances and secrete chemokines that attract more leukocytes
Are there many types of macrophages?
yes, they are given specific names based on function and tissue location

Granulocytes
A group of four cell types characterized by cytoplasmic granules of enzymes
inflammatory molecules and hormones that are released in response to infection.
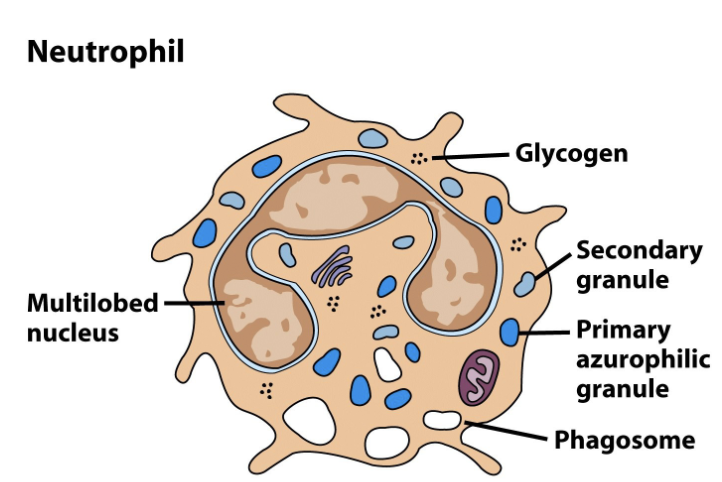
Neutrophils
how common are they?
morphology?
how do they engage with pathogens?
what do they respond to?
line of defense?
lifespan?
Most numerous leukocyte (50%-70%) and numbers increase quickly in response to infection (leukocytosis → high “white blood cell count”)
Morphology = multi-lobed nucleus (3-5 lobes) with granules & pseudopods
Engage pathogens via rapid and efficient phagocytosis and exocytosis of antimicrobial compounds, including lysozyme
Highly responsive to chemokines released by other leukocytes or by damaged tissues; neutrophils arrive at a damaged site within minutes
Simple, efficient and disposable, neutrophils are the first line of defense and use “swarm tactics” against pathogens
Very short lifespan (1-2 days) but produced in high numbers
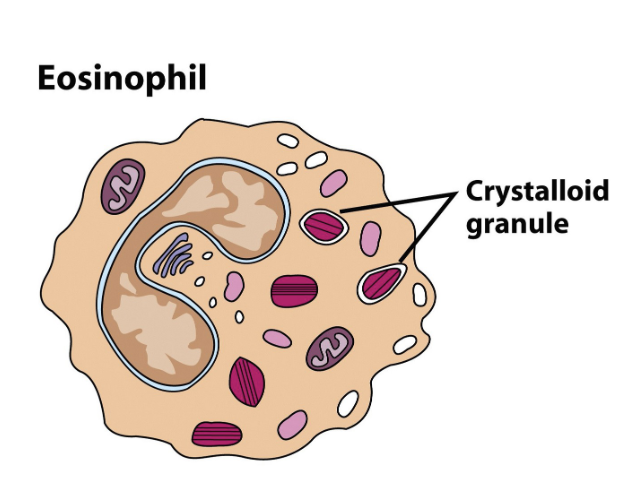
Eosinophils
common?
what are they designed to fight?
morphology?
phagocytic activity?
allergies?
present in low numbers (1%-3%); motile leukocytes designed to fight multicellular parasites (helminths) by releasing digestive enzymes onto them
Morphology: bilobed nucleus & granules
Very minor phagocytic activity; not often employed
Cause of allergies in areas free of worm parasites
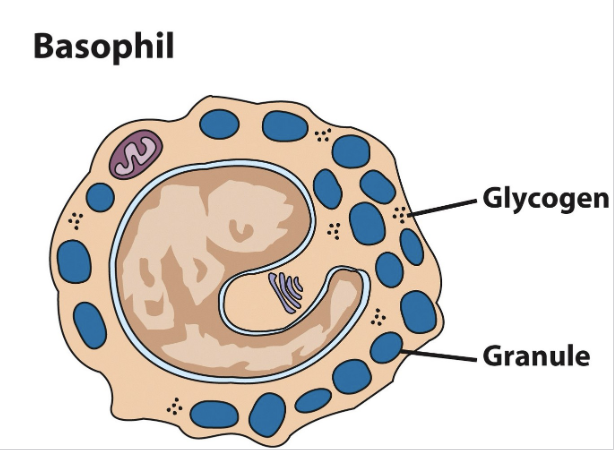
Basophils
function?
what do they respond to?
regulatory?
Morphology?
common?
Release granules of inflammatory chemicals, serotonin & histamine, which increases vasodilation & blood flow to an area (recruits more leukocytes)
Responds in particular to helminths and ectoparasites (ticks); in their
absence reacts to pollen & other allergensSeems to be more of a regulatory cell, designed to attract & direct other leukocytes
MORPHOLOGY = Comma-shaped nucleus and granules, no pseudopods
Least common leukocyte (0.5-1.0% of total WBCs)
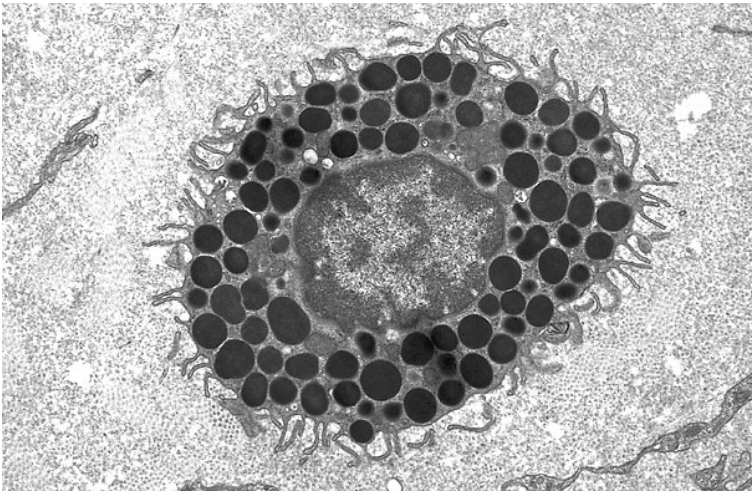
Mast Cells
function?
function of the granules?
what do they cause? what can they aid in?
Morphology?
Circulate in the blood as undifferentiated cells, then enter the tissues and “implant” in a fixed location
Have cytoplasmic granules containing histamine, which are released in response to either chemical signals or physical stimulation (nerve signals)
Cause anaphylaxis, itchiness, hives, etc.
Also aid in wound healing and other processes
Morphology: mononuclear, very high concentrations of granules, pseudopods (sensory, not phagocytic)
Lymphoid Cells
how many types stem from these?
how common are they?
three types of cells that stem from a common progenitor
collectively 20%-40% of blood WBCs and 99% of lymph WBCs
1 trillion cells circulating in average adult human - lymphoid cells = 1% of cells in human body
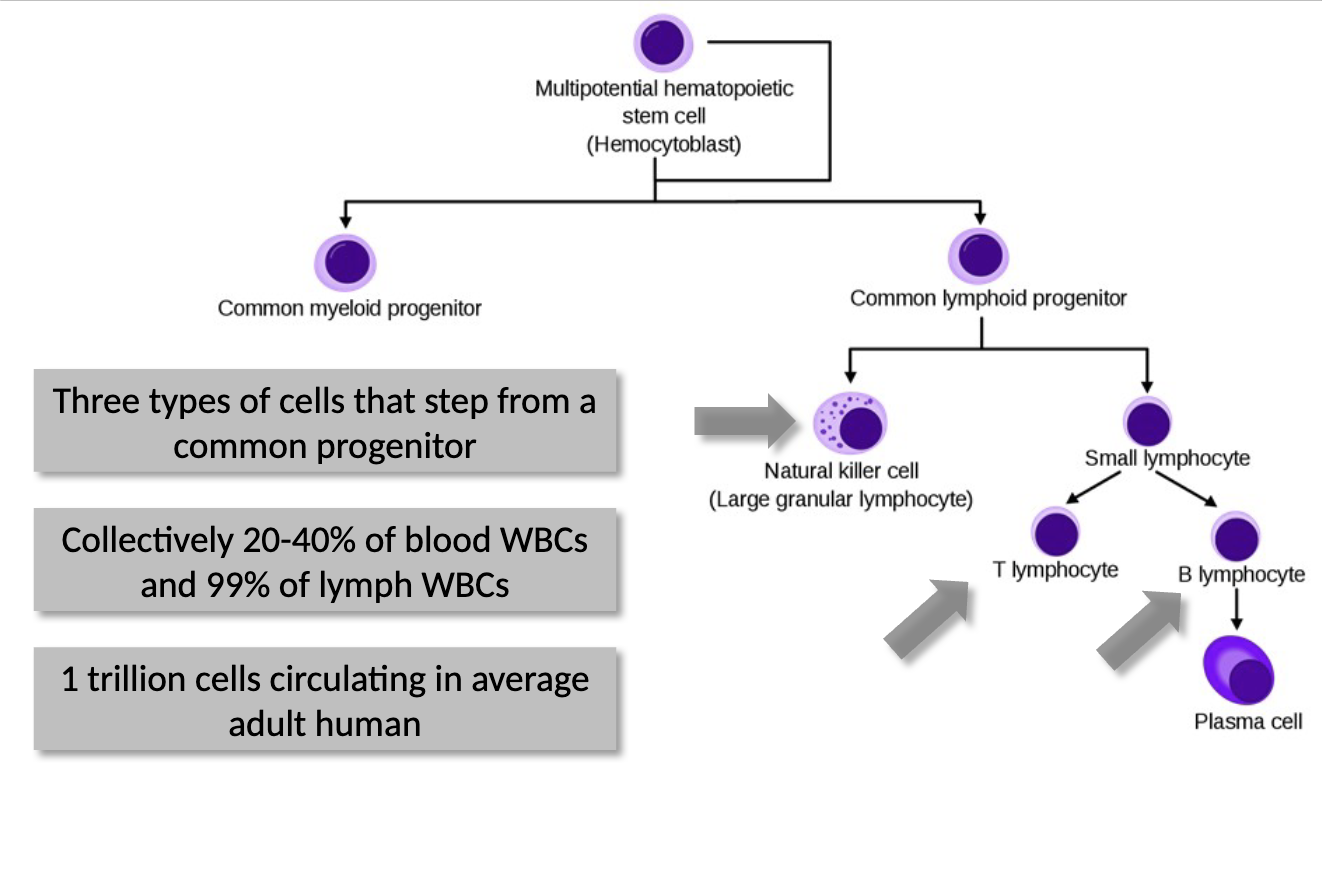
B-Cells
where do they originate? when do they get their receptor assigned?
defining feature?
Naïve B-cell function?
plasma cell function?
memory cells?
B-cells mature in the bone marrow; assigned their specific B-cell receptor
Defining feature = BCR (membrane-bound Ab, 150,000 per cell) can only be detectable chemically, not visibly
Naïve B-cells bind Ag, clonally expand via mitosis, and differentiate into plasma cells and memory cells
Plasma cells secrete 1000 Ab/sec, but have no membrane-bound Ab (live for 1-2 weeks, then apoptosis)
Memory cells are morphologically indistinguishable from naïve B-cells
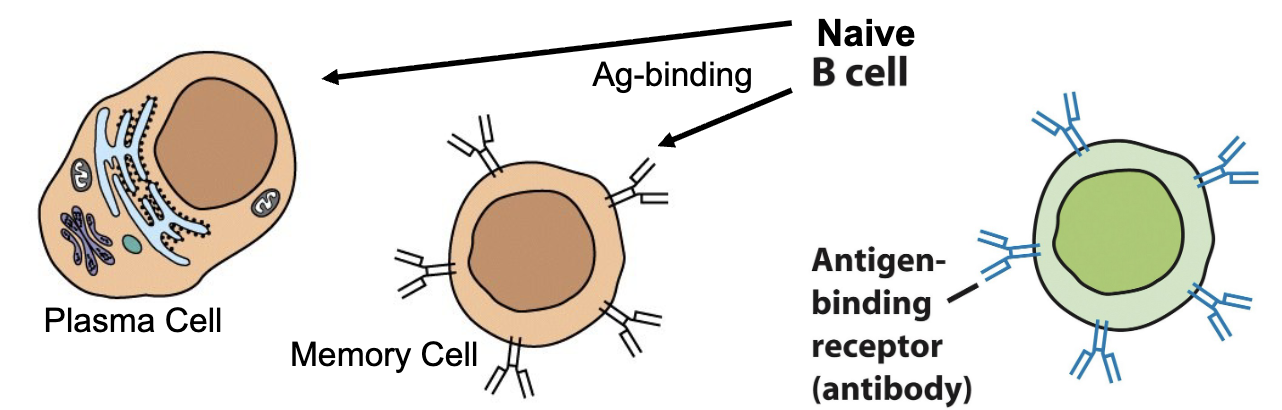
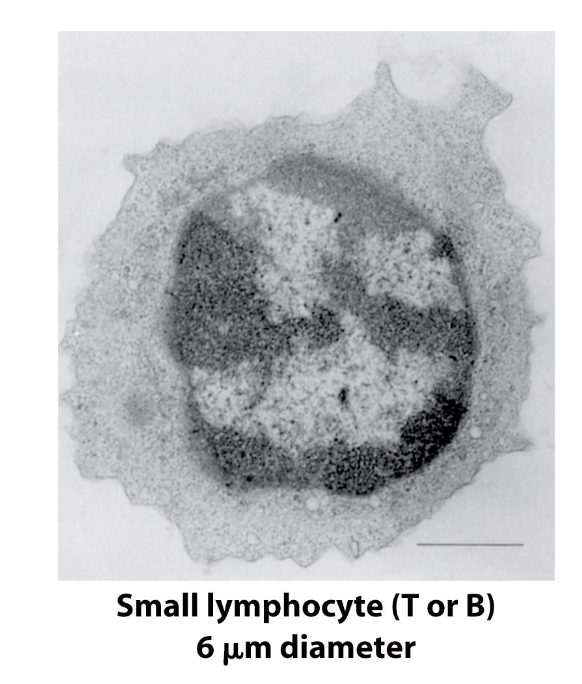
B-cell and T-cell Morphology
size? why?
cost-saver?
what phase are they stuck in?
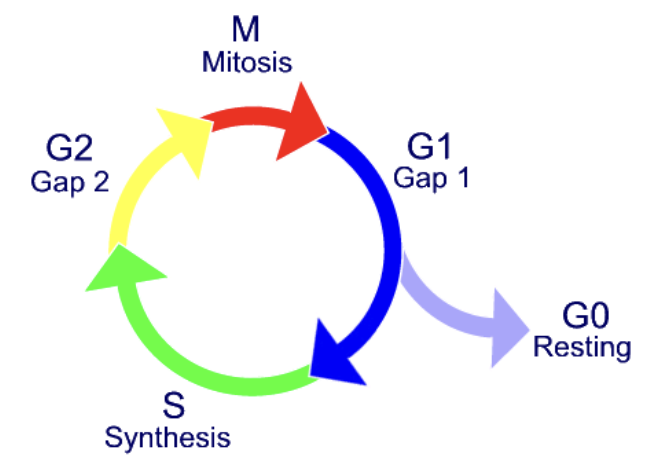
Naïve B-cells and T-cells are morphologically indistinguishable from each other, but are often called small lymphocytes because of their small size (~6 um)
This size is due to low # of organelles and generally low infrastructure (cell is mostly nucleus)
A cost-saving mechanism → no need to develop many organelles because naïve cells are dormant until they bind Ag
Naïve cells are stuck in G0 phase
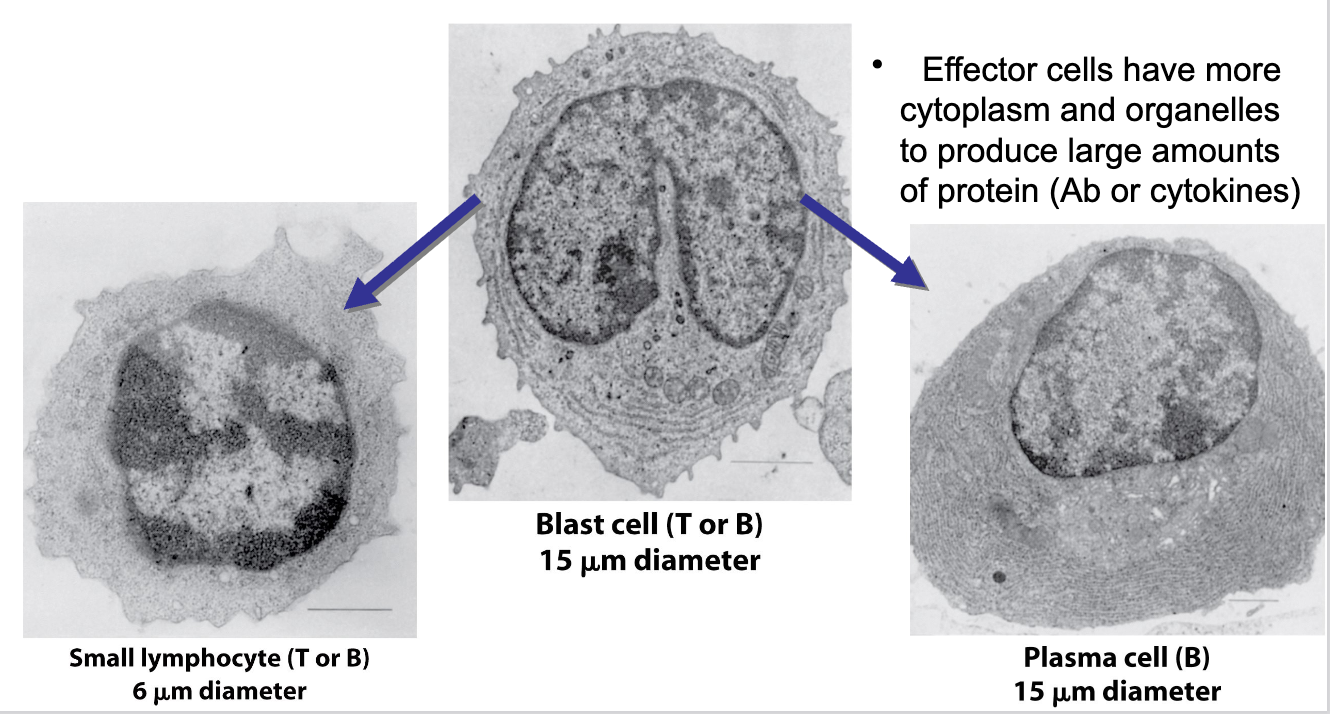
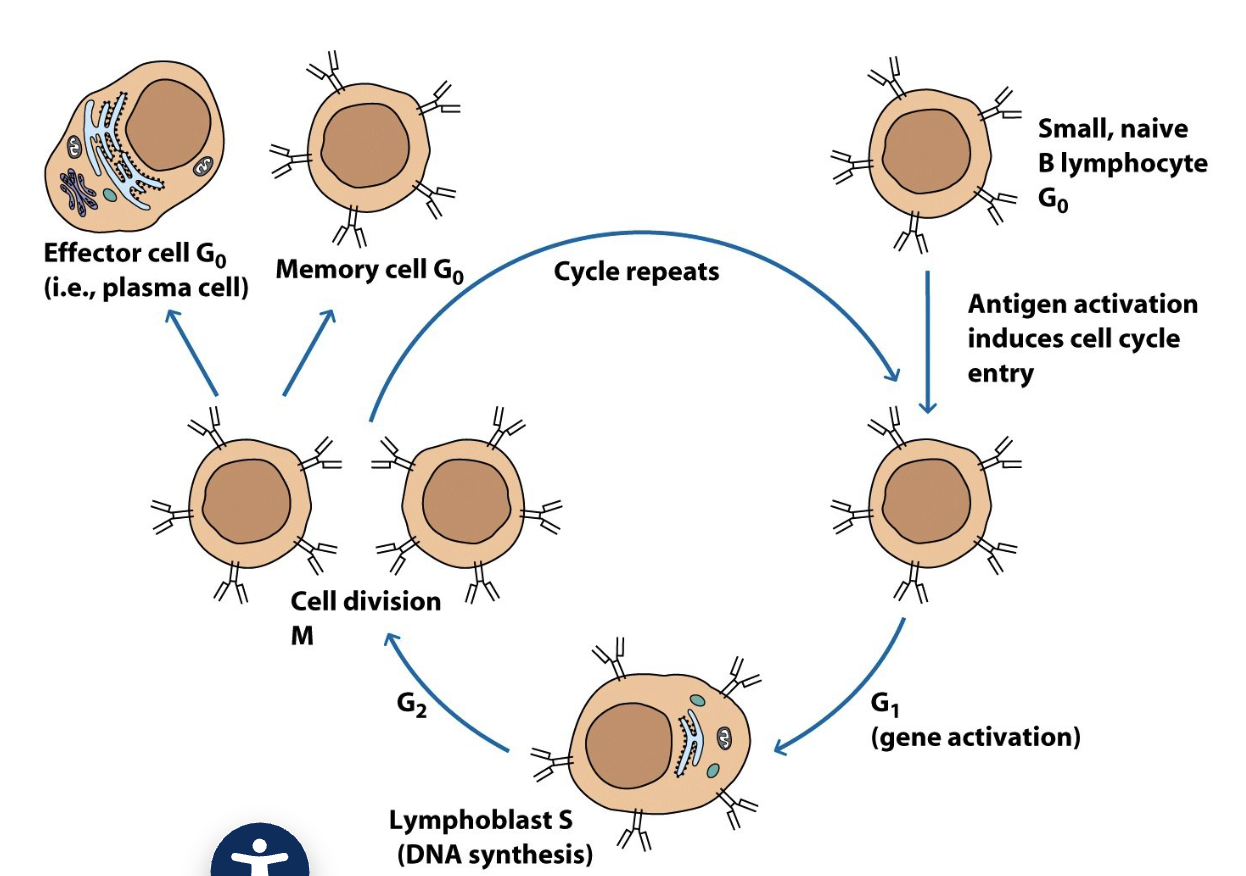
Lymphocyte Differentiation
what happens when small lymphocytes encounter Ag?
what do lymphoblasts do? What happens when they stop dividing?
what do effector cells have more of?
when small lymphocytes encounter Ag, they reenter the cell cycle and grow into a larger lymphoblast (~15 um) that has more extensive organelles
the lymphoblast divides many times and some cells cease dividing, becoming effector cells (plasma cell, or cytokine-secreting effector T-cell)
Effector cells have more cytoplasm and organelles to produce large amounts of protein (Ab or cytokines)
image applies to both B-cells and T-cells
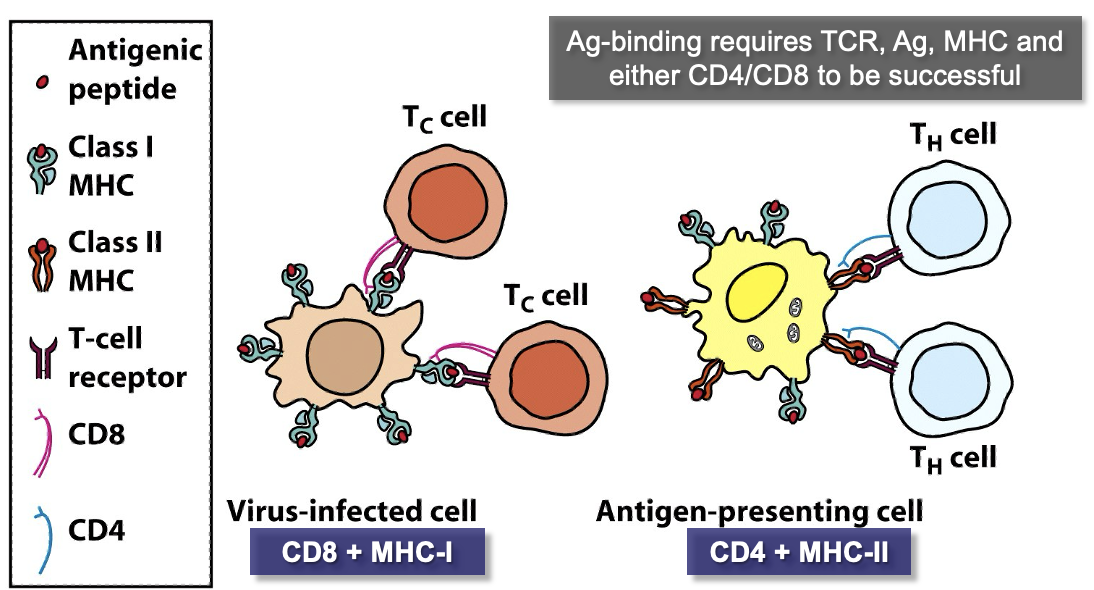
T-cells
difference between naïve T-cells and B-cells?
where do T-cells mature? where is the TCR assigned?
How do TCRs bind?
How many MHC classes are there?
Naïve T-cells are similar to B-cells in many ways except that they have the TCR instead of membrane-bound Ab
A T-cell matures in the thymus gland and is assigned its Ag-specific TCR
TCR can only bind Ag peptide fragments presented in an MHC receptor by an Ag-Presenting Cell (APC)
There are 2 classes of MHC receptors, one for TH cells and one for TC cells.
MHC Class I
expressed by (nearly) all animal cells.
When a cell becomes infected with a virus, it displays viral Ag bound by MHC-I as a “warning”.
TC cells detect these Ag/MHC-I complexes
MHC Class II
Expressed only by antigen presenting cells (APCs).
when certain leukocytes engulfs a pathogen, it displays Ag bound by MHC-II on its surface and is called an APC
T-Cells
what do TH cells bind to and do?
what do TC cells bind to and do?
which glycoproteins do each type of T-cell use? what are their functions?
TH cells bind Ag/MHC-II on APCs, then start secreting cytokines
TC cells bind to Ag/MHC-I on virally infected cells; then differentiate into CTLs and initiate apoptosis in infected cells
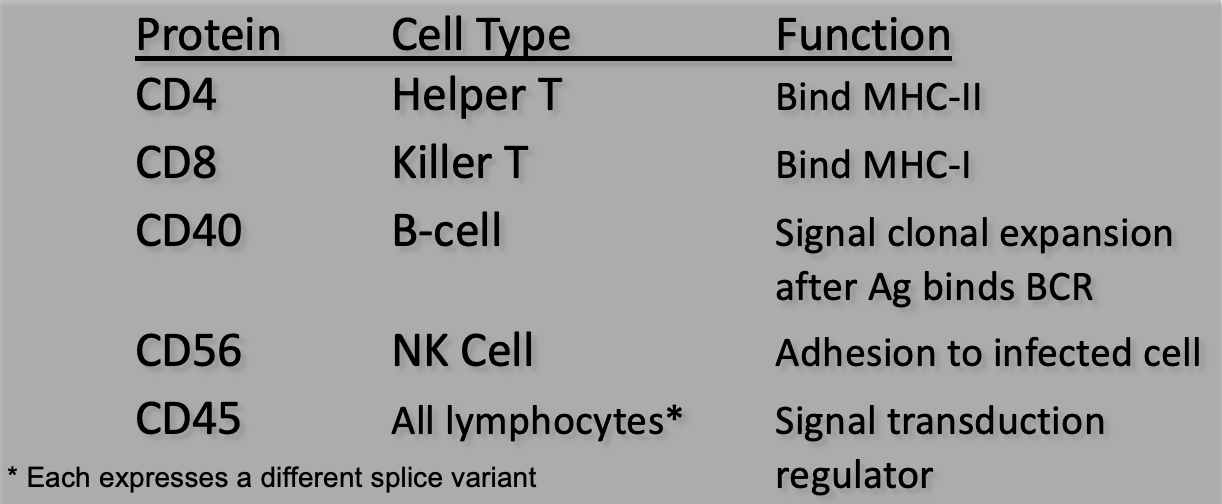
TH cells have the glycoprotein CD4 that enables them to recognize Ag bound to MHC-II only; TC cells have CD8 that recognizes only MHC-I
T-Cell Subtypes
what are TH cell subtypes typically involved in? what do they secrete?
What do regulatory T-cells secrete? what are they important for?
TC cells have only one major type, but TH cells have multiple subtypes
Several subtypes are involved in response to specific types of pathogens & secrete types of pro-inflammatory cytokines suited to fighting a certain pathogen
Regulatory T-cells secrete anti-inflammatory cytokines and are important in ended an immune response or preventing an improper immune reaction
Ag-Presenting Cells
what 3 types of cells can be APCs?
What do B-cells present to?
what do MØs present to?
Only three types of cells can be APCs: Macrophages (MØs), B-cells & Dendritic Cells (DCs)
B-cells bind Ag & present it to T-cells before they undergo expansion & differentiation into plasma cells
MØs present to T-cells after phagocytosis, DCs after pinocytosis
Most Ag is presented in MHC-II to a TH cell, but viral Ag is presented in MHC-I to “kickstart” TC expansion & differentiation into CTLs
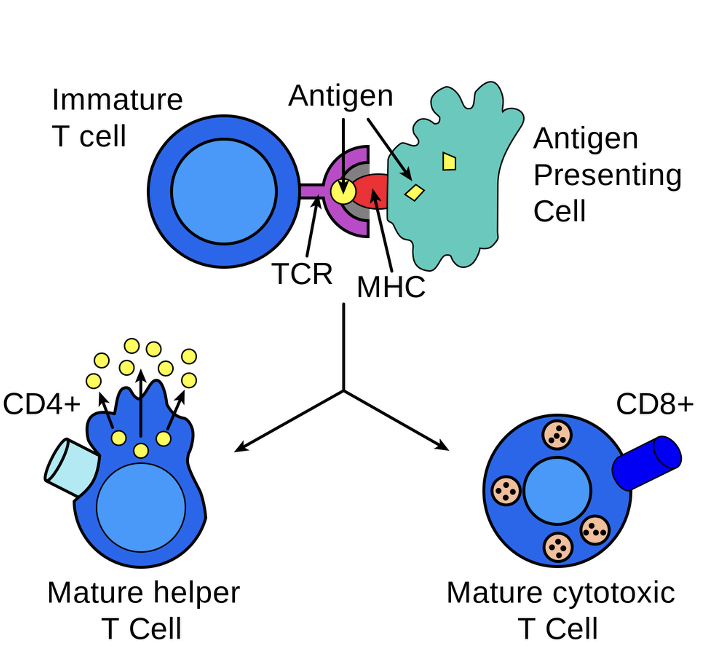
Lymphocyte Cell Surface Molecules
are the surface proteins the same at every stage?
what are the surface proteins denoted as?
what functions do CD proteins serve?
are they specific to one cell type?
Lymphocytes of a particular type, at a given stage of development, have specific glycoproteins on their cell surface
These proteins are denoted CD for cluster of differentiation (old term)
Each CD protein has a specific cell function (often signaling)
Some CD proteins are specific to one cell type, others are found on several
Natural Killer Cells
do they bind specific Ag?
what is their function?
what are the two methods of detection?
what are natural killer t-cells?
Although lymphoid cells, these do not bind specific Ag and are part of the innate immune system
NK cells roam & perform “surveillance” to detect abnormal cells (virally) infected or cancerous) & destroy them
Two methods of detection:
Detect abnormalities on the cell’s surface (lack of normal receptors like MHC-I, or tumor-specific markers)
Bind antiviral or anti-tumor antibodies on the cell surface
Morphology = large, granular lymphocyte, pseudopods for “touching” cell surfaces
Natural Killer T-cells (NKT) are a subgroup with TCRs that detect lipid and glycolipid Ag
Dendritic Cells
what kind of extensions do they have?
where are they found? what is their function?
how do they handle pathogens?
what happens when they engulf pathogens?
where do they present Ag?
Have long, membranous extensions, similar to nerve cell dendrites; very flexible and able to extend/retract
Found in the tissues of major organs, filtering/sampling blood & lymph as it flows through tissues
Engulf pathogens by phagocytosis or pinocytosis (can “drink” its own volume in fluids every hour)
When they engulf pathogens, dendritic cells become APCs
As APCs, they move from tissues into the bloodstream
Move to the lymphoid tissues (e.g. lymph nodes) to present Ag to T-cells
Hematopoiesis
DCs can derive from myeloid or lymphoid progenitors
Subtle differences btw myeloid and lymphoid DCs
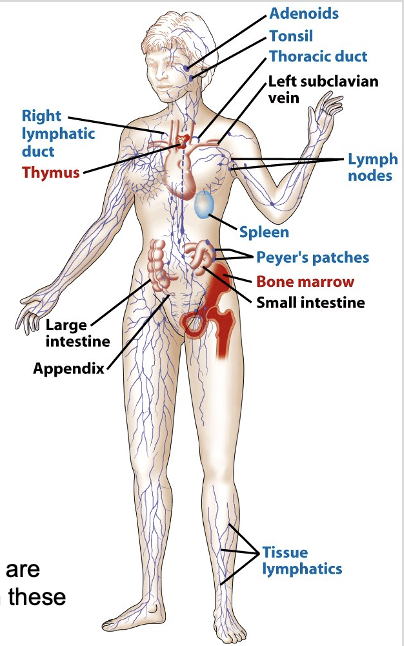
Immune System Organs
what are the primary lymphoid organs? the secondary?
what are their functions?
why are the blood and lymphatic system important?
PRIMARY LYMPHOID ORGANS: are sites where lymphocytes mature and gain their specific Ag-receptors
bone marrow
thymus
SECONDARY LYMPHOID ORGANS: provide sites where lymphocytes and Ag are forced together
lymph nodes
spleen
mucosal lymphoid tissue
The blood stream and lymphatic system are critical for moving cells & Ag to and from these organs
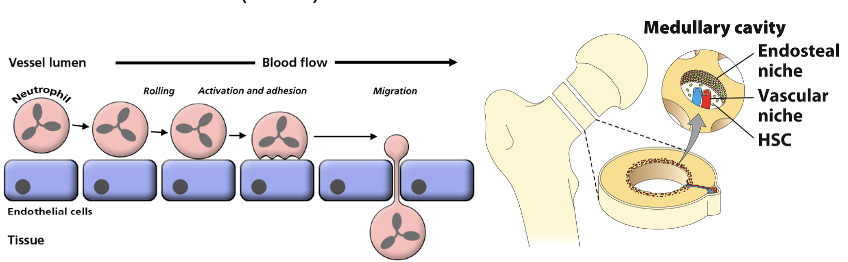
Bone Marrow
what are stromal cells? function?
what are osteoblasts? function?
where do differentiated cells go?
what is unique about immune cell cytoskeletons?
Stromal cells express soluble cytokines & membrane-bound proteins that promote HSC proliferation & differentiation (a stem cell niche)
Osteoblasts in the endosteal niche produce bone tissue and also direct the mitosis & differentiation of HSCs
As cells differentiate, they migrate into the vascular niche and enter blood vessels
Immune cells have very pliable cytoskeletons to extravasate in and out of blood vessels; cell adhesion molecules (CAMs) assist
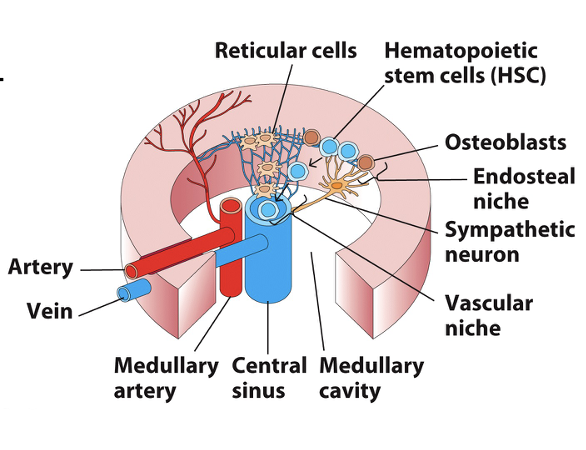
Bone Marrow
what are reticular cells? function?
what are sympathetic neurons?
where do all leukocytes (except T-cells) mature?
how does maturation occur?
How do B-cells develop? where do they mature?
Reticular cells form connections between osteoblasts of the endosteal niche and endothelial cells lining blood vessels
Sympathetic neurons stimulate/regulate release of blood cells
All leukocytes, except T-cells, fully mature in bone marrow
Maturation occurs on a gradient as cells proceed towards blood vessels
B-cells are assigned their specific Ag-receptor (BCR) as they develop & self-reactive B-cells are destroyed
B-cell maturation occurs in the bone marrow for humans and mice, but not other animals
Fetal spleen and gut lymphoid tissue are common sites
Thymus Gland
what is the thymus
what are T-cells produced from?
what are lobules made of?
what are thymocytes? where are they? why do they diminish?
Bi-lobed, surrounded by a capsule and divided into lobules
T-cells are produced from HSCs in the bone marrow, but mature here
Each lobule has an outer cortex, containing many immature T-cells (thymocytes), and an inner medulla, which contains sparse thymocytes
Thymocytes arrive in the cortex and then migrate inward to the medulla, departing via medullary blood vessels
Thymocyte numbers diminish due to negative selection that destroys those that are potentially self-reactive
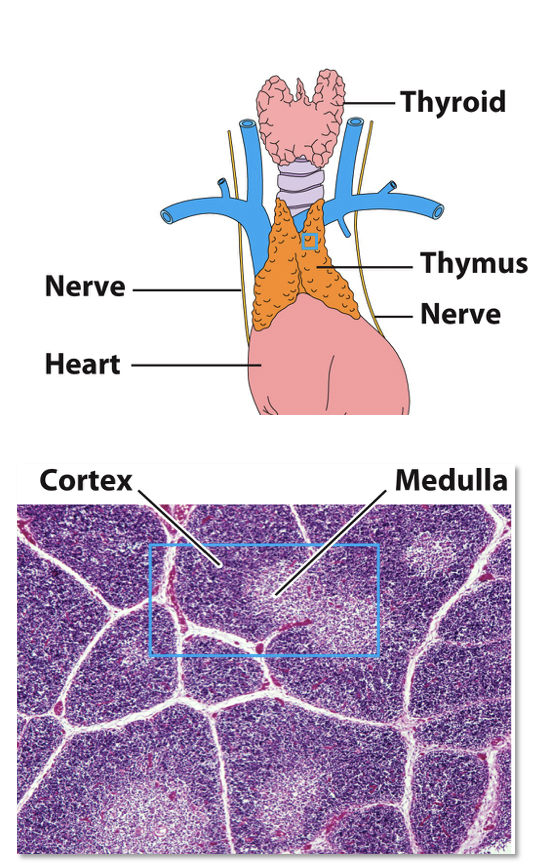
Thymus Gland
what happens as T-cells mature?
what do cortical and medullary thymic epithelial cells do?
what happens to self-reactive thymocytes?
what do Hassall’s corpuscles do?
As T-cells mature in the thymus, they begin expressing their Ag-specific TCR
Cortical and medullary thymic epithelial cells (TECs) & dendritic cells interact with thymocytes, promoting development & testing them for self-reactivity
Self-reactive thymocytes undergo apoptosis and macrophages engulf the resulting apoptotic bodies
Surviving thymocytes development into fully mature T-cells
Hassall’s corpuscles secrete cytokines to aid development and possibly test thymocytes for self-tolerance
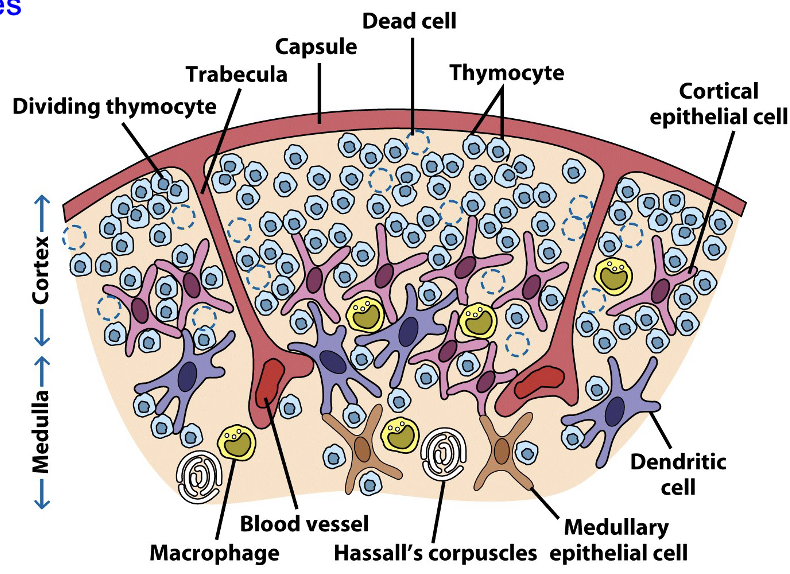
T-Cell Maturation Overview
95% of thymocytes fail selection, either because the TCR doesn’t bind Ag/MHC properly or because it cross-reacts with self-Ag
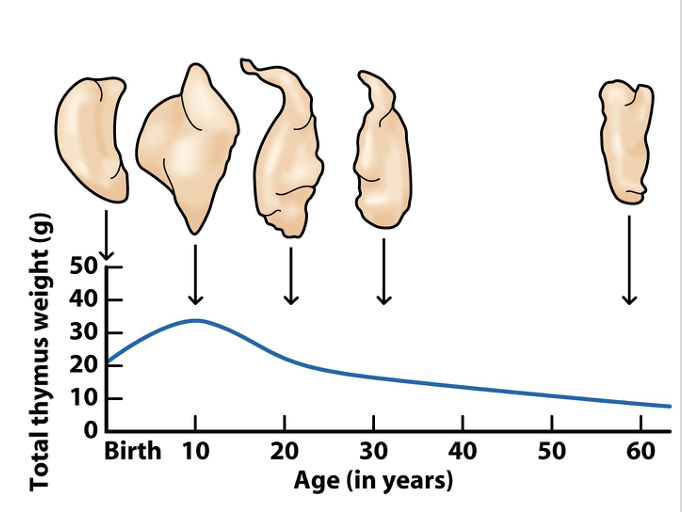
The thymus atrophies with age and T-cell production slows (at age 65, only 2% T-cell production)
The thymus is one of several glands (e.g. tonsils, appendix) once thought to be vestigial & useless because function wasn’t understood