Test 1 Study Guide
Chem Review
Understand atomic structure
Atoms are composed of protons (+ charged particles), neutrons (neutral particles), and electrons (- charged particles)
Protons and neutrons are in the nucleus
Electrons are in the orbitals surrounding the nucleus
Describe how different types of bonding work (ionic, hydrogen, covalent - polar and nonpolar)
Differences in electronegativity dictate how electrons are distributed in covalent bonds
Electronegativity: a measure of how strongly an atom pulls shared electrons toward itself in a bond
Covalent Bond: when two atoms share electrons
Hydrogen Bond: an attraction between a hydrogen atom with a partial positive charge and another atom, with a partial negative charge
Non-polar covalent bonds: share electrons equally, hydrophobic
Polar covalent bonds: don’t share electrons equally, hydrophilic
An atom in a molecule with a high electronegativity holds electrons more tightly (partial negative charge), and the other atom will have a partial positive charge
Ionic bonds result when electrons are transferred from one atom to another to give both atoms full valence shells
An ion is an atom or molecule that carries a charge and comes from polar covalent bonds
cation: an atom that loses an electron and becomes positively charged
anion: an atom that gains an electron and becomes negatively charged
Explain what makes structures polar and nonpolar
Become polar when their atoms have unequal electron sharing due to differences in electronegativity, creating partial positive and negative charges, and the molecules geometry is asymmetric which causes the charge difference to not cancel out
Are nonpolar if the atoms share electrons equally (low electronegativity difference) or if the molecule is symmetric and the individual bond polarities cancel each other out
Identify and name the 7 functional groups (table 2.3)
Amino: −NH2
Carboxyl: —COOH or —CO2H
Carbonyl: —C=O
aldehydes: R-CHO
ketones: R-CO-R’
Hydroxyl: —OH
Phosphate: —O-P=O-O
Sulfhydryl: —SH
Explain the properties of water that make it a great solvent
Solvent: any liquid in which one or more solids or gases can dissolve
Water’s small size, highly polar covalent bonds, and bent shape resulting in overall polarity are unique
Has covalent OH polar bonds
Cell Parts
Compare and contrast eukaryotic and prokaryotic cells
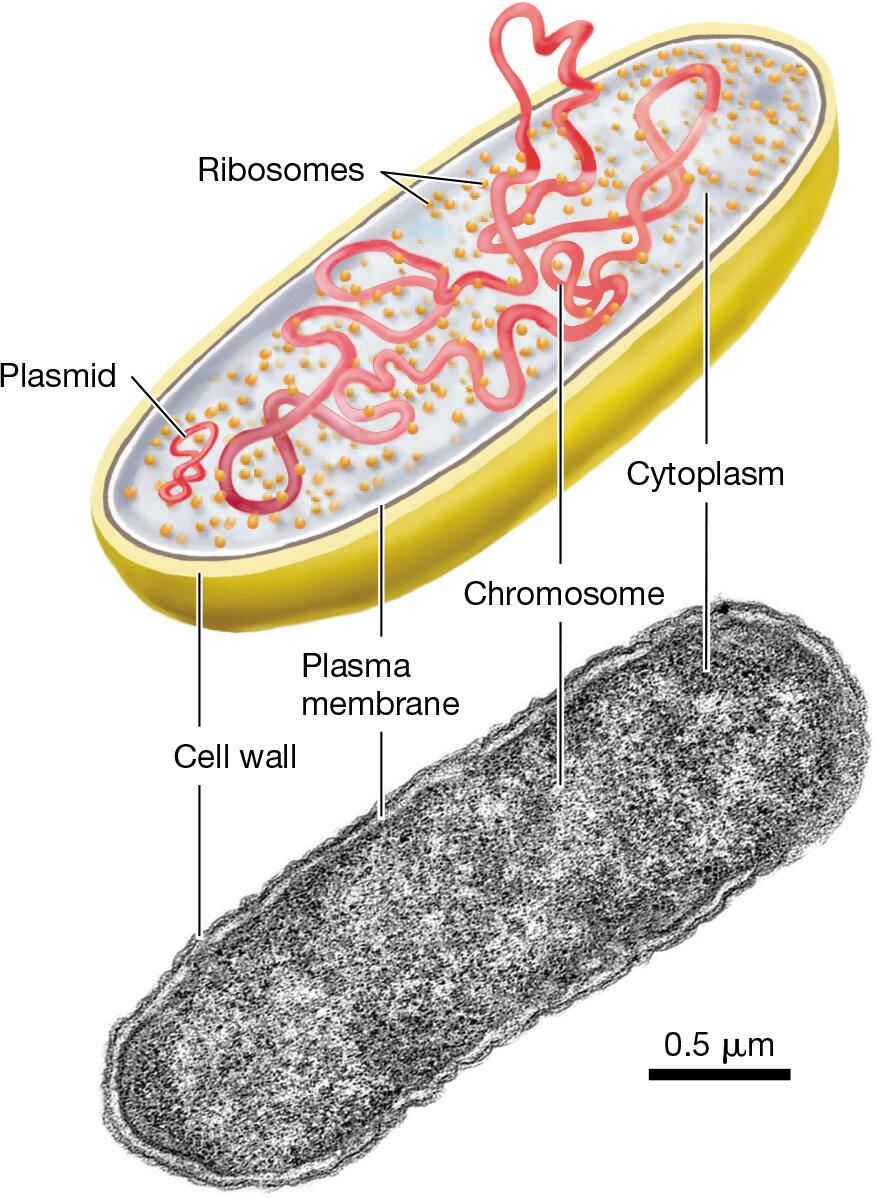
Prokaryotic Cells: absence of a membrane-bound nucleus and lacks membrane bound organelles
characteristics include a plasma membrane, at least one chromosome, many protein-synthesizing ribosomes enclosed within a plasma membrane
Eukaryotic Cells: presence of a membrane-bound nucleus that contains the cells genetic material (DNA) and other membrane-bound organelles like the mitochondria, endoplasmic reticulum; building blocks for complex organisms including plants, animals, fungi, and protists
Similarities: have DNA, cell or plasma membrane, cytoplasm, ribosomes
Differences: cell size, cell structure, organization, DNA structure, DNA location, cell division, ribosome size, cytoskeleton, reproduction
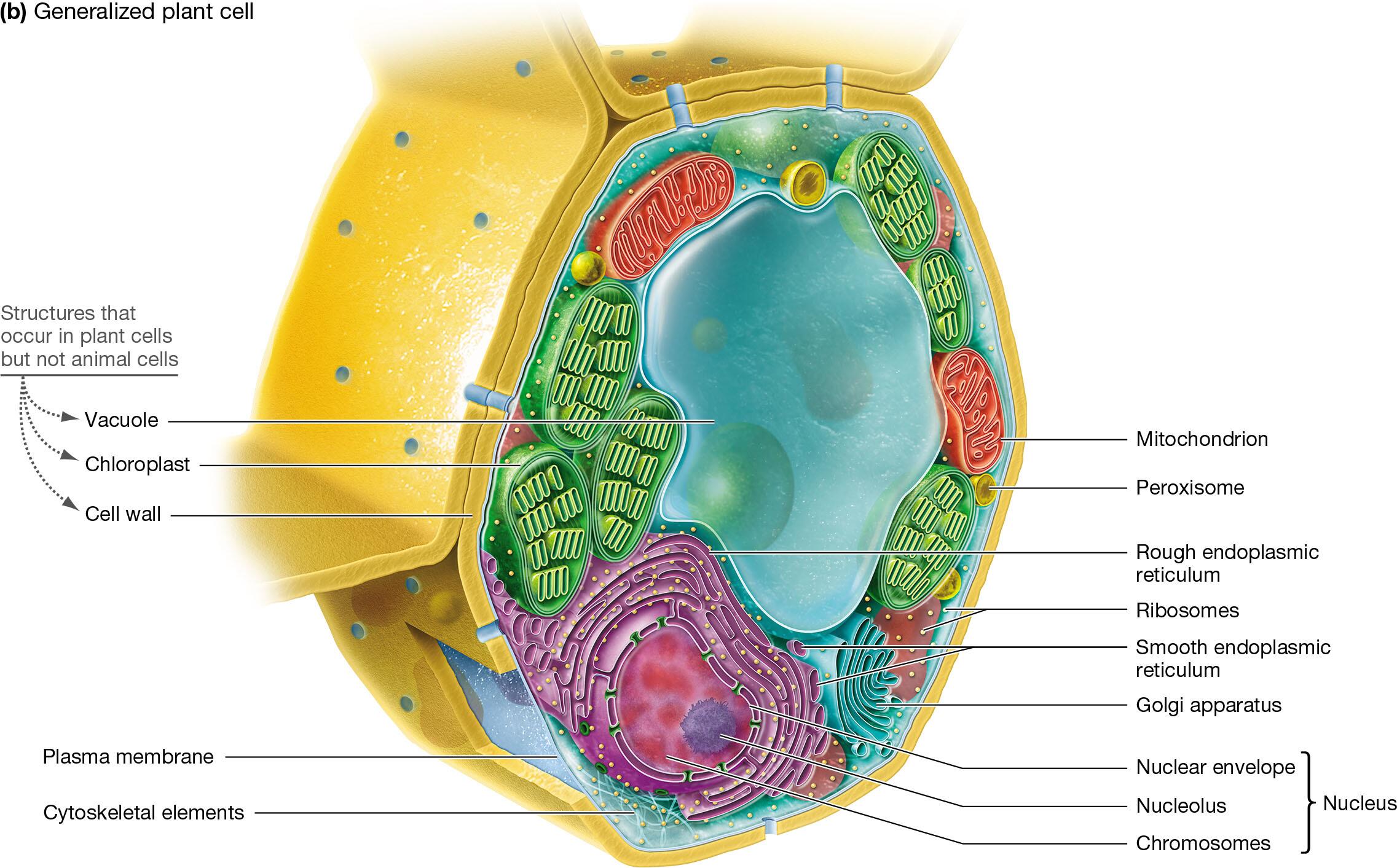
Compare and contrast animal and plant cells
Plant and animal cells are both eukaryotic cells
Similarities: have nucleus, cell membrane, cytoplasm, mitochondria, endoplasmic reticulum, Golgi apparatus, ribosomes, peroxisomes
Differences:
cell wall - plant cells have a rigid outer layer made of cellulose that surrounds the cell membrane which provides structural support while animal cells do not have a cell wall and the cells are contained by a flexible membrane
chloroplasts - present in plant cells since it is the site of photosynthesis, but it is absent in animal cells since they obtain energy from consuming other organisms
vacuoles - plant cells have a large central vacuole that stores water, nutrients, waste, maintains turgor pressure to keep the cell rigid where animal cells have numerous small and temporary vacuoles which are involved in removal and transport instead
storage - plant cells store carbohydrates as starch and animal cells store carbohydrates as glycogen
shape - plant cells have a fixed rectangular or cubic shape due to the rigid wall where animal cells have an irregular or round shape giving them more flexibility
Provide a brief description for each of the organelles and parts of the eukaryotic and prokaryotic cells (7.2)
Eukaryotic Cell
nucleus: information storage and transmission, ribosome subunit assembly, structural support
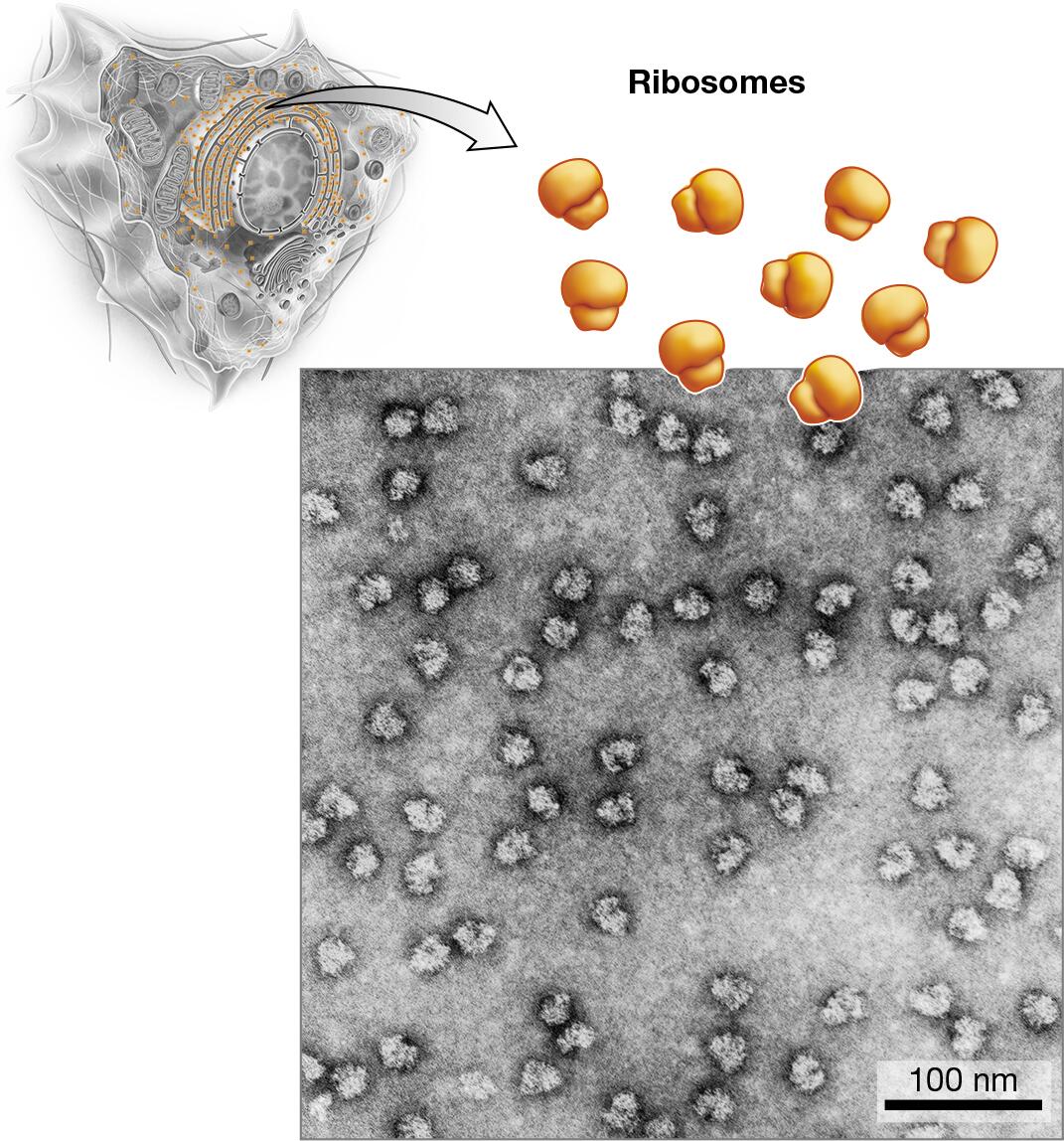
ribosomes: protein synthesis
endomembrane system: smooth ER, rough ER, Golgi apparatus, lysosomes
rough endoplasmic reticulum: protein synthesis and processing
smooth endoplasmic reticulum: lipid synthesis and processing
Golgi apparatus: protein, lipid, and carbohydrate processing
lysosomes: digestion and recycling
vacuoles: digestion and recycling
peroxisomes: oxidation of fatty acids, ethanol, or other compounds
mitochondria: ATP production
chloroplasts: production of sugars via photosynthesis
cytoskeleton: structural support, movement of materials, in some species movement of whole cell
plasma membrane: selective permeability, maintains intracellular environment
cell wall and extracellular matrix: protection, structural support
Prokaryotic Cell
nucleoid: central region of the cell that contains the single circular chromosome of DNA
ribosomes: sites of protein synthesis, responsible for translating mRNA into proteins, floating freely in the cytoplasm
cytoplasm: site for many metabolic reactions, jelly-like substance that fills the cell and holds all the other components
cell membrane: outer covering separates the cells interior from the outside environment and regulates what enters and exists the cell
cell wall: rigid layer outside the cell membrane that provides structural support, protects the cell from the mechanical damage and helps maintain its shape
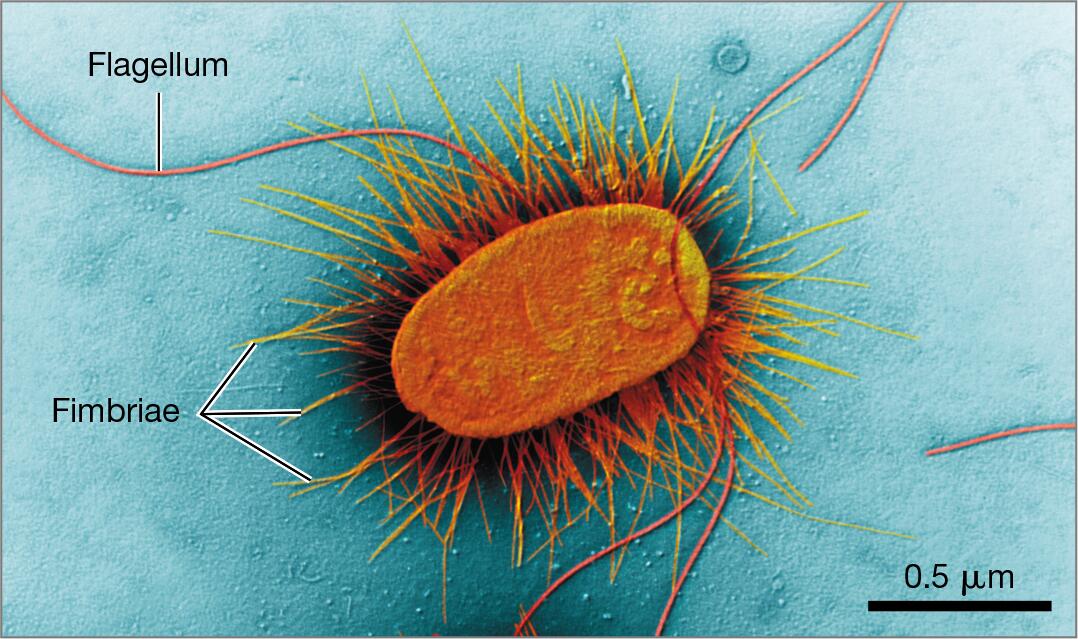
capsule, flagella, pili, plasmids
Identify the organelles of a cell in different models or pictures


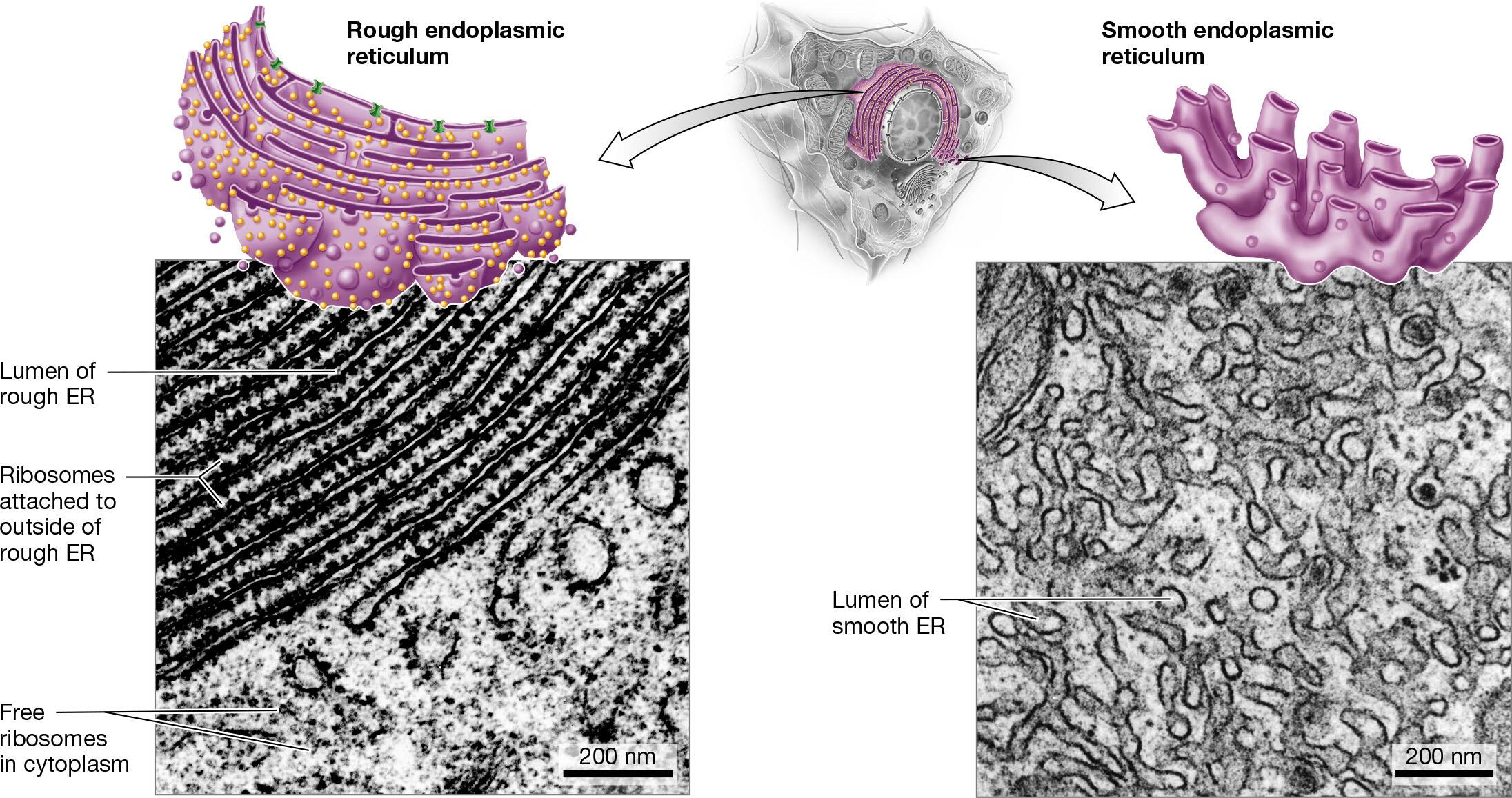
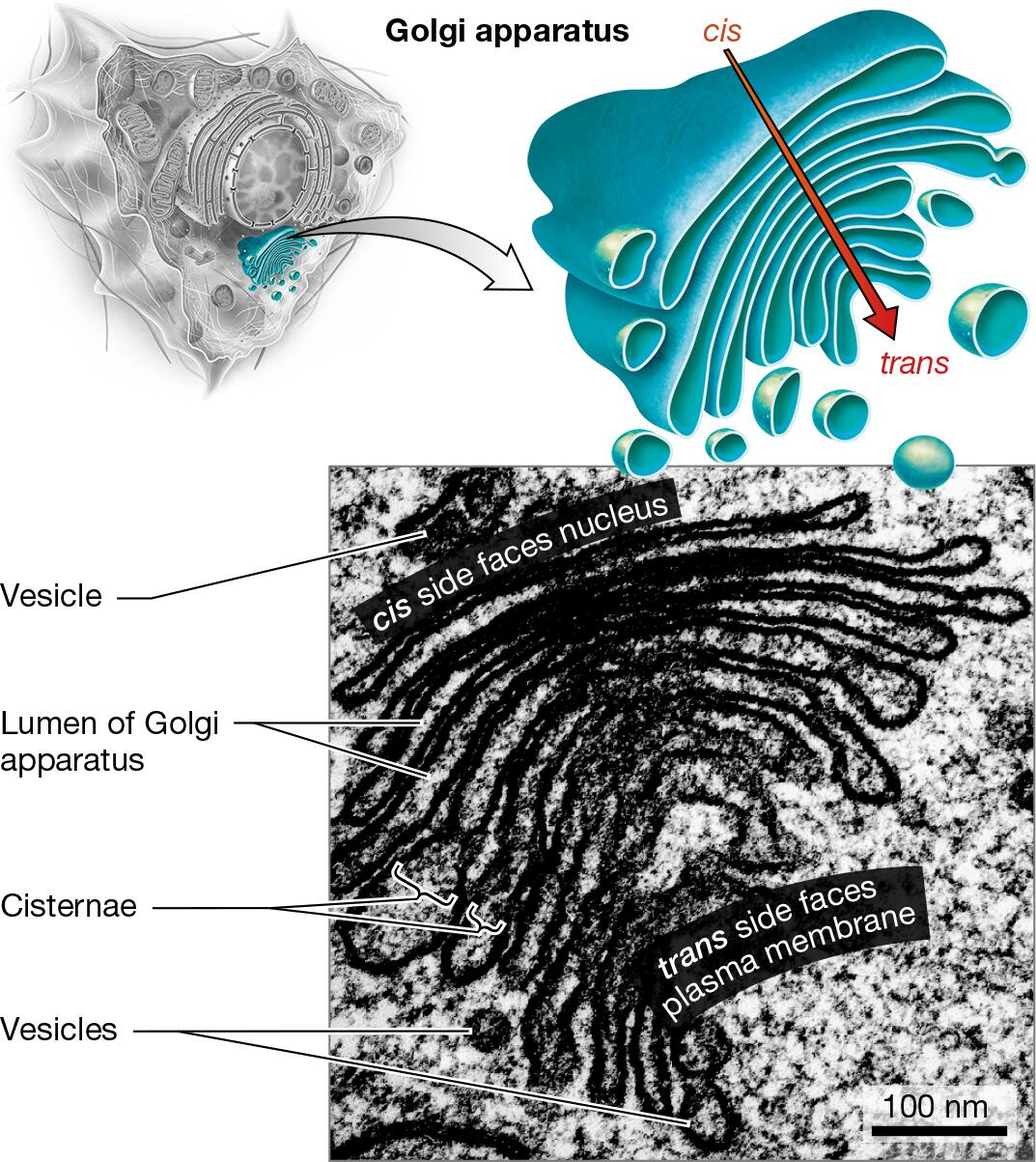
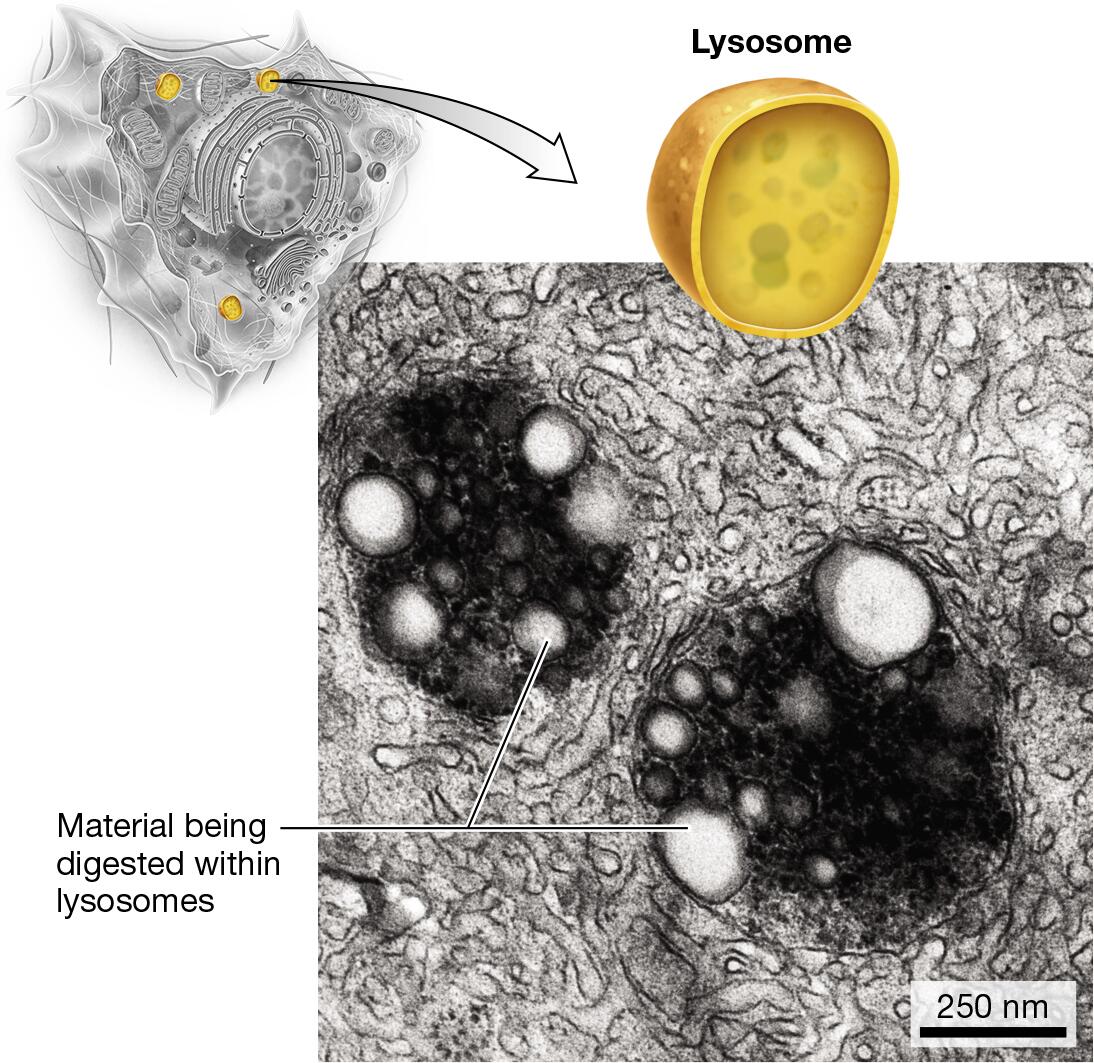
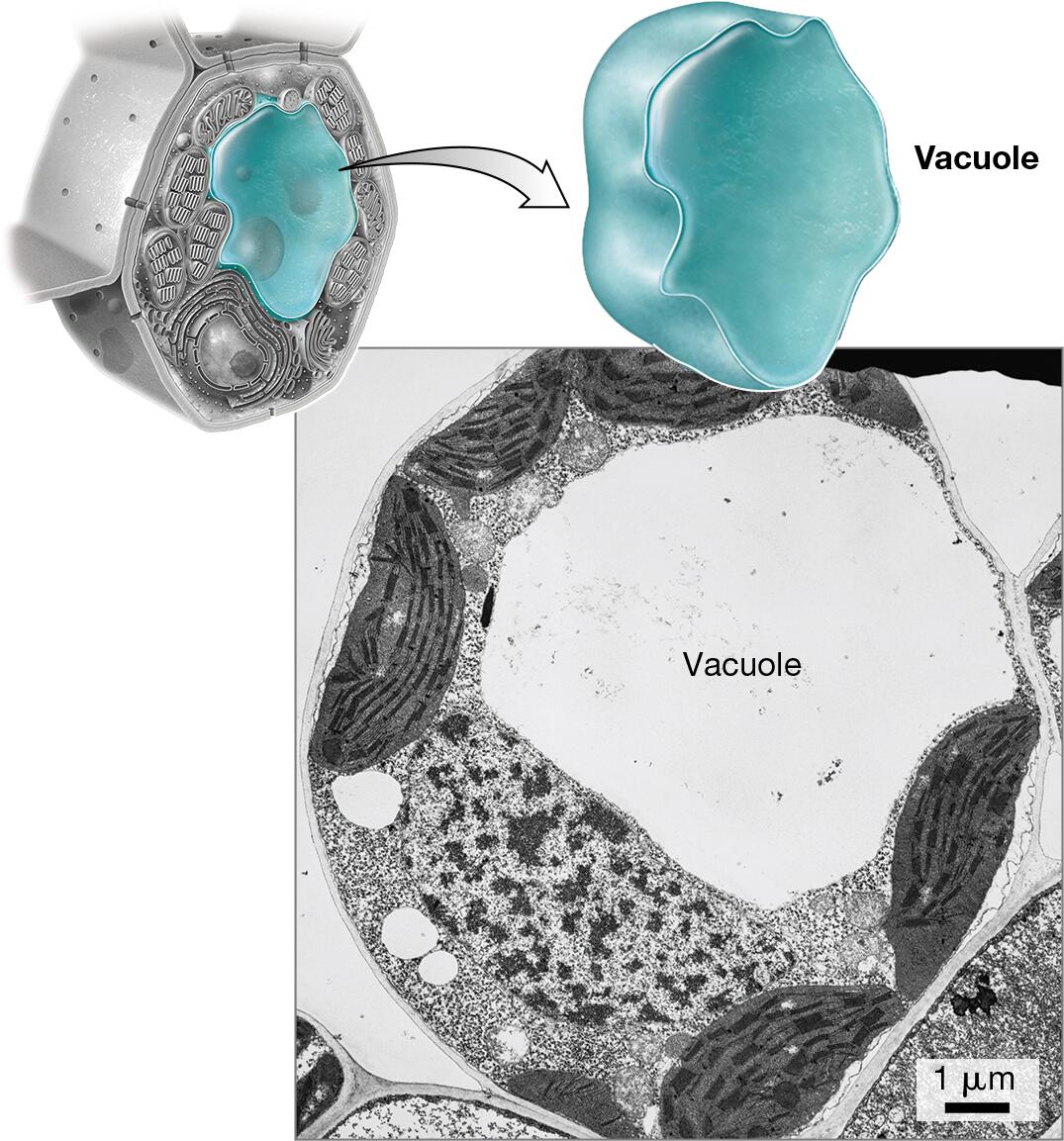
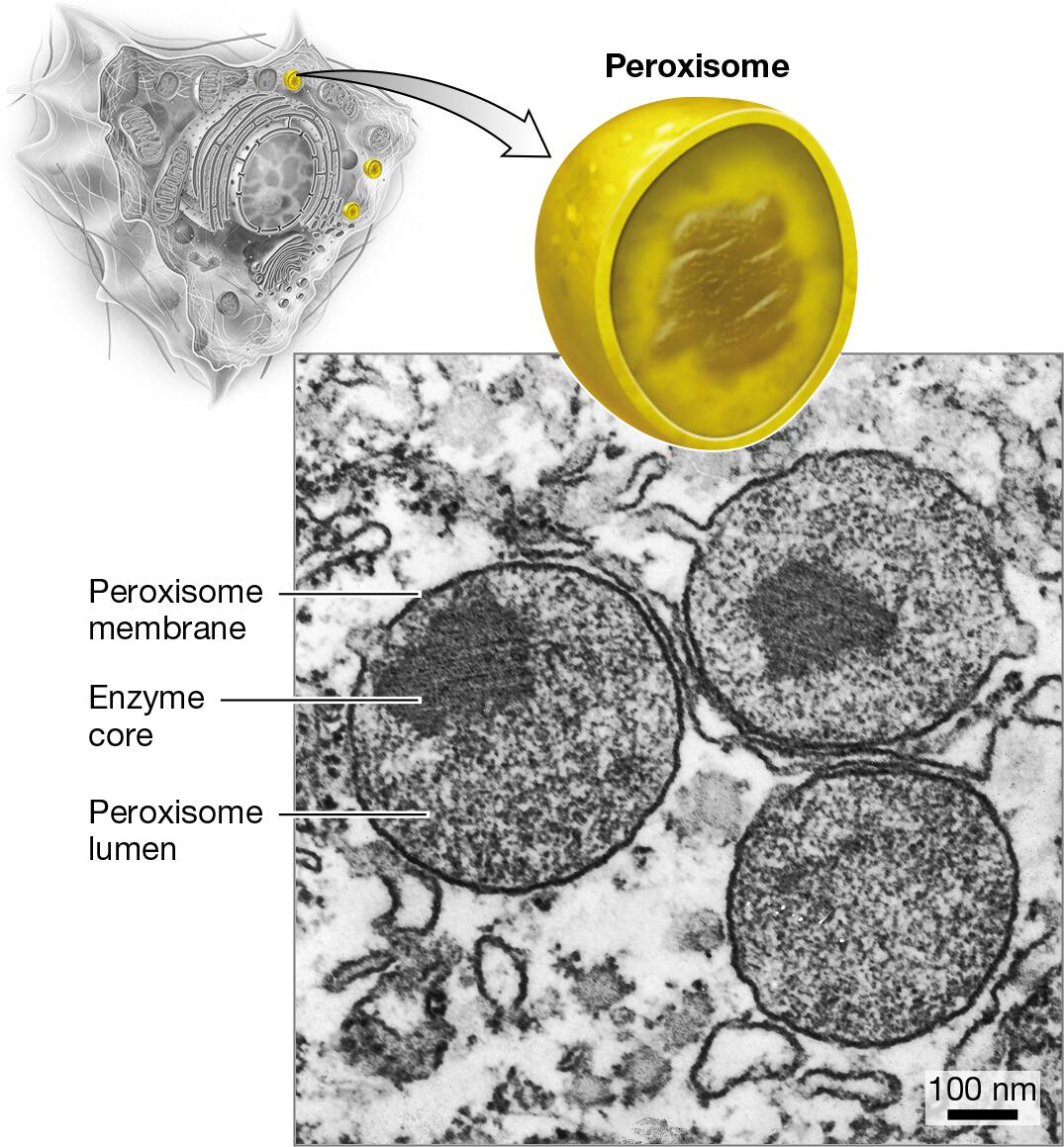
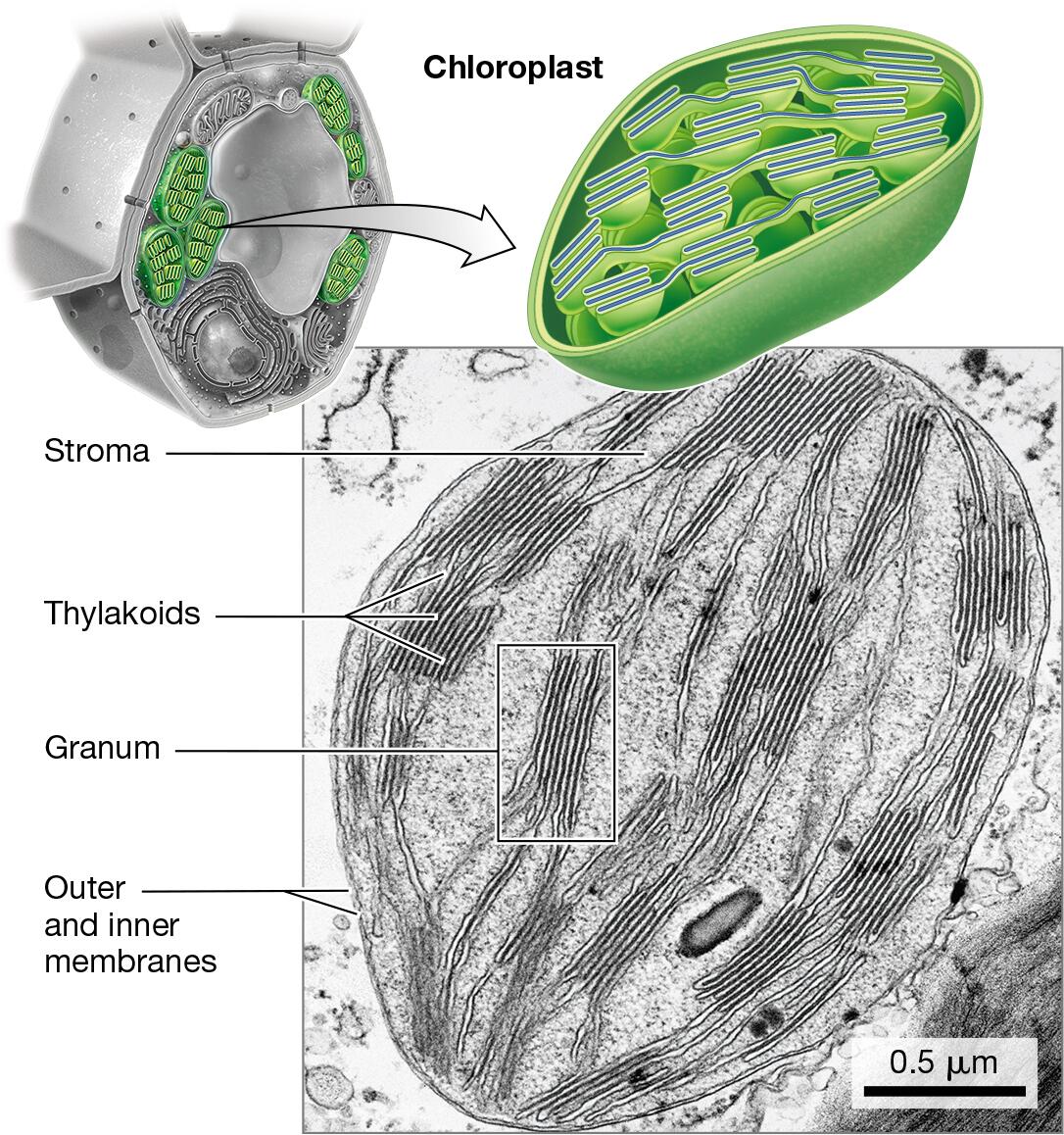
Explain how the relative amounts of organelles can indicate function of the cell
Cells that perform a certain task at a high volume will possess a greater abundance or a larger size of the organelle responsible for that task
Carbohydrates
Know the building blocks for carbohydrates
Monosaccharides: simple sugars and monomers of carbohydrates
vary in carbon count (either 3, 5, 6)
vary in spatial arrangement of atoms
monosaccharide (one monomer), oligosaccharide (small polymers), polysaccharide (many large polymers)
Identify the structure of carbohydrates
(CH2O)n
Carbonyl group that serves as one of the monosaccharides is either at the end of the molecule forming an aldehyde/aldose or within the carbon chain forming a ketone/ketose (C=O)
Contain carbonyl group (C=O), hydroxyl group (O—H), carbon-hydrogen bonds (C-H)
carbonyl and hydroxyl groups are polar, carbohydrates are hydrophilic
Points on structures are carbon (know for images on test)
Nam the type of linkage associated with carbohydrates
Glycosidic linkages: a covalent bond formed by a condensation reaction between two sugar monomers; joins the resides of a polysaccharide
connects monomers together
forms between hydroxyl groups
a-linkage —» energy molecule
b-linkage —» structural molecule
Identify the polysaccharides and their functions in various organisms
Starch: a-glucose monomers, to store energy in plants
Glycogen: composed of glucose monomers joined by a-1,4 and a-1,6 glycosidic linkages; major form of stored carbohydrates in animals
Cellulose: composed of glucose monomers joined by b-1,4 glycosidic linkages; found in the cell wall of algae, plants, and some bacteria of fungi
Chitin: composed of N-acetly-glucosamine (NAG) monomers joined end to end by b-1,4 glycosidic linkages; found in cell walls of fungi and many algae, and in external skeletons of insects and crustaceans; structural and protective function
Peptidoglycan: found in bacterial cell walls; provides structural support, rigidity, and protection to the cell
Similarity between starch and cellulose: both made of glucose
Difference between starch and cellulose: starch has a-linkages while cellulose has b-linkages, starch is a helix molecule while cellulose is linear composed of parallel strands with hydrogen bonds
Compare and contrast the polysaccharides from table 5.1
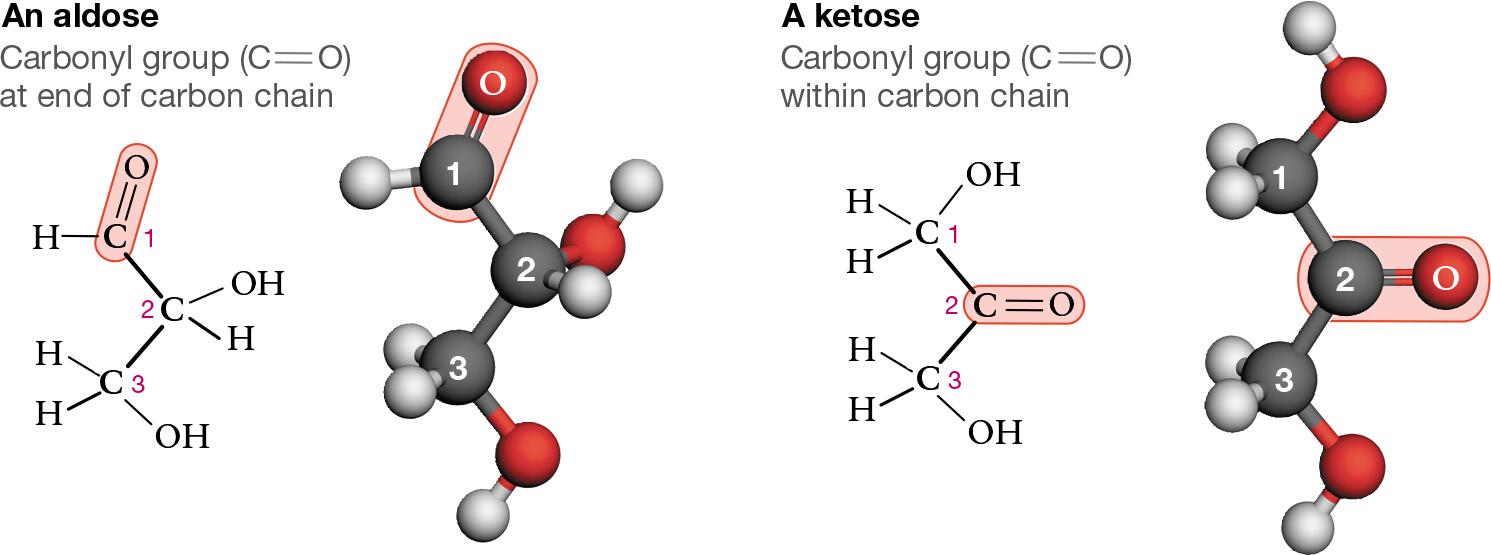
Lipids
Know the building blocks for lipids
Fatty acids and glycerol
fatty acids are long chains of carbon atoms
glycerol is a three-carbon alcohol molecule with 3 hydroxyl groups
Name the type of linkage associated with lipids
Ester linkage
Adding a fatty acid through dehydration reaction makes a triglyceride (stores energy) which makes an ester linkage and a carbonyl
Understand the reaction that attaches building blocks to the macromolecules and how it can be reversed
Dehydration synthesis/condensation reaction
monomers polymerize through this
a chemical reaction in which two molecules are joined covalently with the removal of an —OH from one and an —H from another to form water
Hydrolysis
a chemical reaction in which a molecule is split into smaller molecules by reacting with water, most involve splitting of polymers into monomers
adding a water molecule
water molecule reacts with the bond linking the monomers separating one monomer from the polymer chain
Be able to explain how the number of carbons and type of bonds within the fatty acid affect the characteristic of the lipid
Identify the different types of lipids and functions in the cell
Steroids, Fats, Phospholipids
don’t polymerize or make chains
characteristic: non-polar or hydrophobic and have hydrocarbons
Steroids
distinguished by a 4 fused ring carbon skeleton, attached to carbons in the ring
Fats
composed of three fatty acids linked to a glycerol molecule, also called triglycerides, primary role is to store energy, form by a dehydration reaction (a hydroxyl group of glycerol and the carboxyl group of a fatty acid), glycerol and fatty acid molecules become joined by an ester linkage
Phospholipids
consist of a glycerol linked to two fatty acid tails and a phosphate group with an ester linkage
hydrophilic head that contains glycerol and a hydrophobic tail that is composed of two non-polar fatty acids
in bacteria and eukarya, isoprenoids in archaea
primary role is to form a lipid bilayer of all cell membranes which creates a protective semi-permeable barrier that regulates the passage of substances into and out of the cell
Explain the amphipathic nature of phospholipids
when amphipathic lipids are placed in water the hydrophilic heads interact with water, the hydrophobic tails interact with each other away from the water
forms lipid micelles and lipid bilayers
Discuss which types of molecule would be permeable through the lipid membrane
Explain the factors that can alter membrane permeability
Know the structure of cholesterol and how it impacts both permeability and fluidity
Explain the setup and the trends associated with figure 6.10