Unit 5 - Kinetics
1/42
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
What is true about half-life for 1st order reactions?
It does not change throughout the experiment as reactants deplete.
What is the equation to find half-life for first order reactions?
ln(2)/k=t1/2
k = rate constant
t1/2= half-life
What is the equation to find half-life for second order reactions?
t1/2 = 1/-k[A]0
What is the equation to find the half-life for zero order reactions?
t1/2 = [A]0/2k
What determines how quickly or slowly a reaction occurs?
Kinetics do, which is the study of the rates of a reaction.
What is an average rate of a reaction?
It is the rate of change of product/reactant concentration over a specific time interval.
How is the rate of change of either product/reactant determined?
It is determined by the stoichiometry in the balanced chemical equation.
What influences the rate of a reaction?
Reactant concentrations, temperature, surface area, catalysts, other environmental factors.
How do you usually find the average rate of change for either a product/reactant?
It is usually the change in [product/reaction] / change in time.
D + 3E —> 2F
When the chemical reaction is carried out under certain conditions, the rate of dissapearance of D is 2.5×10-2 Ms-1. What is the rate of dissapearance of E and F under these same conditions?
Using the rate given, you would multiply it by 3E/1D to get rate of dissapearance of E. You would do the same thing to find F (2F/1D). This will be the rate of appearance of F.
Answers: 7.5 × 10-2 Ms-1 (E)
Answer: 5.0 × 10-2 Ms-1 (F)
What is collision theory?
It states that the rate of a reaction is influenced by anything that effects the number of forces/collisions like: temperature, reactant concentrations, catalysts, or surface area.
What happens to the rate of the reaction if we manage to increase the concentration of reactants or decrease its volume?
Collisions will go up in both cases and therefore have a higher reaction rate.
What happens to the rate of the reaction if we manage to increase the temperature of reactants?
The rate of the reaction will increase because particles are moving around faster.
What is the rate law?
It shows the rate of the reaction as an equation which shows reactant concentration, product concentration, and temperature.
How can we determine rate law?
You can use many experiments at varying concentrations.
Solve for m, n, and k
You can use one experiment over time
Graphing [A] over time and/or using natural log (first order) and/or using 1/[A] (second order)
Use a reaction mechanism
Use stoichiometry coefficients of the reactants from the slowest elementary step mechanism (not from the overall reaction) you have to have a mechanism though!
What does the reaction order tell you?
It tells you how the rate of a reaction depends on each reactant.
What does 0 order tell you?
It tells you that the reactant is independent of the reaction.
What are the units of K for 0 order?
mol/(L * t)
What are the units of K for 1st order?
1/t
What are the units of K for 2nd order?
L/(mol * t)
What are the units of K for 3rd order?
L2/(mol2 * t)
What is the purpose of the integrated rate law?
Helps to answer questions like “How long will it take for x moles per liter A to be used up?” or “What is the concentration of A at y minutes?”
What is half-life?
The time required for the reactant concentration to reach half of its initial value (t1/2).
What are elementary reactions?
It involves actual collisions of particles that result in a single step reaction.
EX: It looks like those columns that gives a mini equation, then another, then an overall.
What is an intermediate?
Something part of an elementary reaction that goes from a product to a reactant in the second elementary reaction.
Why do we exclude intermediates from an overall reaction?
It is because they produced BUT then used up during another reaction.
What is a catalyst?
Something part of an elementary reaction that goes from a reactant in one elementary reaction to a product in another one.
Why are catalysts excluded from the overall reaction?
It is because may be used but are always reproduced. They’re the same at the beginning and end of a reaction. They are not considered part of the overall reaction.
How do catalysts increase the rate of a reaction?
By increasing the number of effective collisions OR providing a reaction pathway with a lower activation energy. (it can only be one or the other!)
How do catalysts work?
They bind with a reactant either through IMFs or covalent bonds. Then, it will increase the reaction by providing an alternative pathway with lower potential energy for an activated complex (lowers activation energy).
Why is the Maxwell-Boltzmann Distribution helpful?
It can make an estimate of the percentage of collisions and then show you what amount of energy is required for a reaction to occur.
What must happen for an elementary reaction to successfully produce products?
Reactants must successfully collide to initiate bond-breaking and bond-making events.
What must happen for a reaction to occur? (hint: 3 things)
Reactants must collide, have proper orientation, and have sufficient energy.
Remember, temperature ____ (does/doesn’t) change activation energy.
doesn’t
Why is it that as temperature increases, rate also increases?
This is because at higher temperatures, more particles on average have enough energy to collide successfully and reach activation energy.
What happens at an activated complex (AKA the peak of the reaction energy profile, AKA the graph that shows exo/endothermic reactions)?
It shows bonds being partially formed or broken.
What happens as activation energy increases? Why?
The reaction rate decreases. This is because a lower percentage of collisions will have sufficient energy to overcome the activation energy to form a product.
What happens as activation energy decrases? Why?
The reaction rate increases. This is because a greater percentage of collisions will have sufficient energy to overcome the activation energy to form a product.
When you increase temperature, what happens in terms of reactions occurring? Why?
More reactions will occur because more particles will have sufficient energy to overcome the activation energy.
What happens to the activation energy when a catalyst is used? Why?
The activation energy decreases, allowing more collisions to result in a reaction. This is because the catalyst provides an alternative pathway with a lower energy barrier for the reaction.
Why is it that you look at the elementary reaction that is the slowest to determine a mechanism? (usually) What do you include in the slowest reaction?
This is beacuse it is a rate-limiter and has the highest activation energy, making it the slowest. You include catalysts.
Make sure to do practice outside of these flashcards. Practice making the multistep reaction energy profiles, actually solving for K, M, and N in rate laws. Doing half-life.
Okay :)
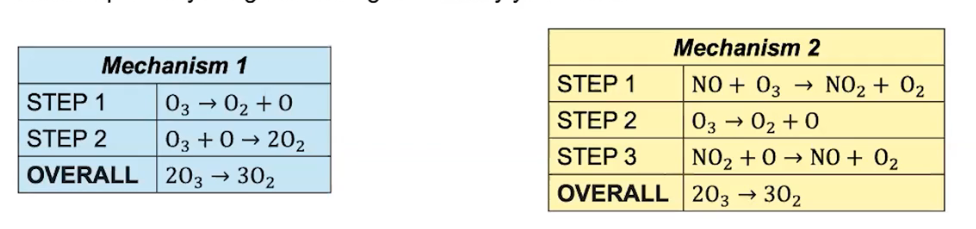
Which reaction would be faster and why?
The second reaction would be faster because it HAS A CATALYST. This catalyst will INCREASE the rate of a reaction and therefore lower the activation energy in mechanism two, allowing more particles to form more quickly.