Shapes of molecules
1/14
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Which experiances the most replusion
LP - LP strongest
LP -BP middle
BP -BP weakest
Whats is the shape, diagram and bond angle in a shape with 2 bonded pairs and 0 lone pairs?
Linear
180 deegres
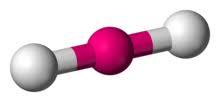
Whats is the shape, diagram and bond angle in a shape with 3 BP and 0 LP
Triagonal planar
120
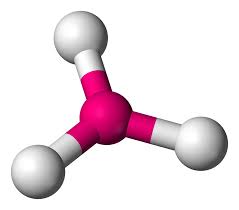
Whats is the shape, diagram and bond angle in a shape with 4 BP and 0 LP
Tetrahedral
109.5
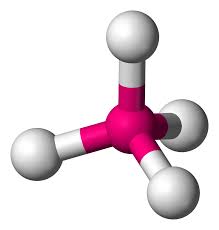
Whats is the shape, diagram and bond angle in a shape with 5 BP and 0 LP
Triagonal bipyrimidal
90 and 120
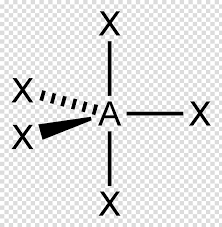
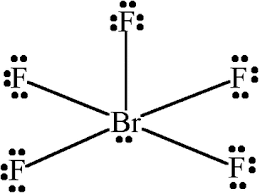
Whats is the shape, diagram and bond angle in a shape with 6 BP and 0 LP
Octrahedral
90
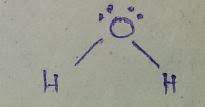
Whats is the shape, diagram and bond angle in a shape with 2 BP and 1LP
Bent (V-shape)
118
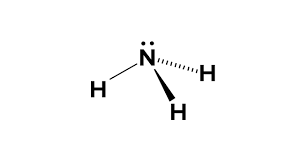
Whats is the shape, diagram and bond angle in a shape with 3BP and 1LP
Pyrimidal
107
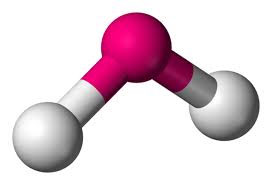
Whats is the shape, diagram and bond angle in a shape with 2BP and 2LP
Bent (V-Shape)
104.5
Whats is the shape, diagram and bond angle in a shape with 4BP and 1LP
See saw
119 and 89
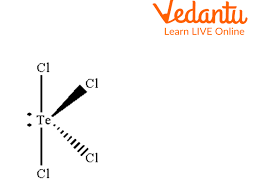
Whats is the shape, diagram and bond angle in a shape with 3BP and 2LP
T-shape
89
Whats does the Electron Pair Repulsion Theory state?
That electron pairs will take up positions as far away from eachother as possible to mimise the repulsive forces between them
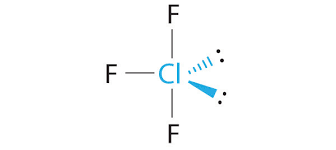
Whats is the shape, diagram and bond angle in a shape with 5BP and 1LP
Square pyrimid
89
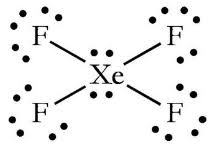
Whats is the shape, diagram and bond angle in a shape with 4BP and 2LP
Square planar
90
What does the shape of molecules depend on?
Number of electrons in the valence shell of the central atom
Number of these elecrtons which are in bonded or lone pairs