BioChem chp 7 carbs and glycoconjugates of cell surfaces
1/150
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
151 Terms
carbs have what general formula
(CH2O)n
monosaccharides are what; broken down into
simple sugars
cannot be broken down into a simpler sugar under mild conditions
oligosaccharides are what
2-10 sugar residues
polysaccharides are
polymers of simple sugars
hundreds to thousands of residues
what is an aldose
has an aldehyde functionality
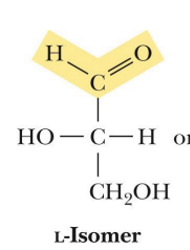
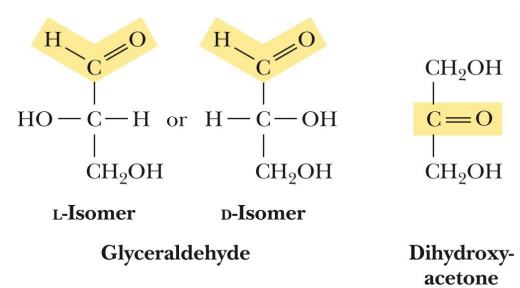
what is a ketose
has a ketone functionality
something being an aldose or a ketose is based on what
location of the carbonyl carbon
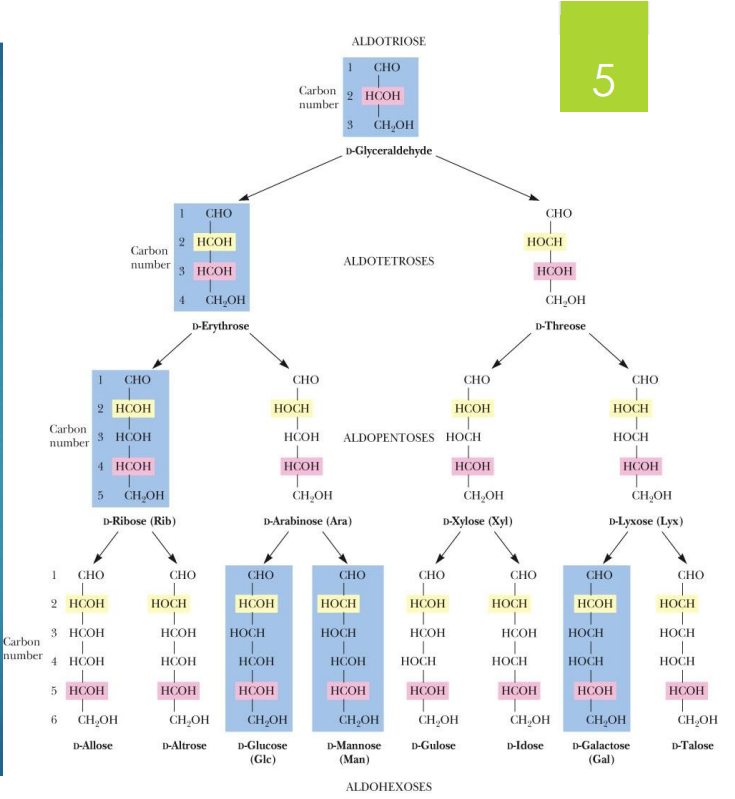
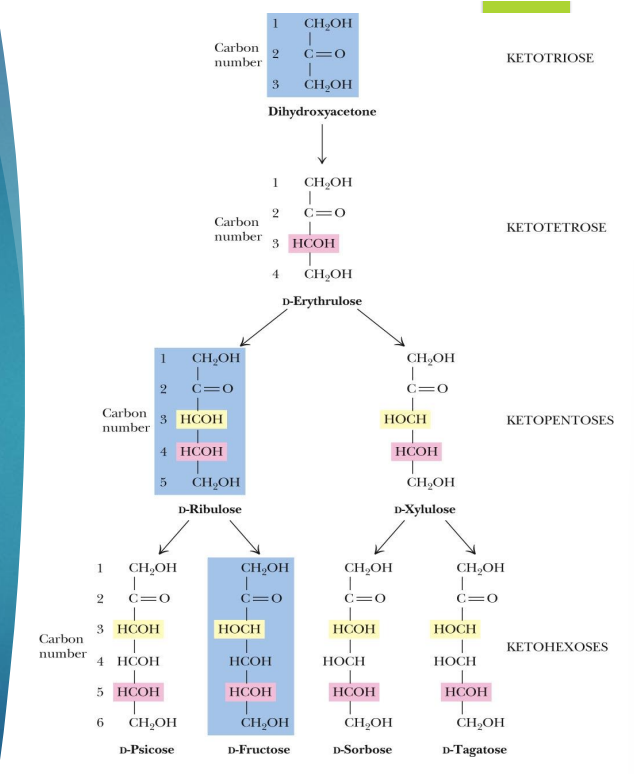
number of carbons in a sugar and name
Triose (3C)
Tetrose (4C)
Pentose (5C)
Hexose (6C)
Heptose (7C)
what are the main aldoses (6)
glyceraldehyde
D-erythrose
D-ribose
D-glucose
D-mannose
D-galactose
what are the main ketoses (3)
dihydroxyacetone
ribulose
fructose
fisher projection and wedge-dash representation
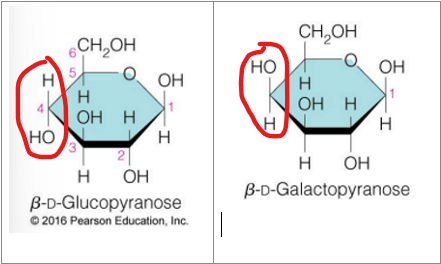
Fischer projections (top) are the most compact way to represent stereochemistry
The wedge- dash representations of D- and L-glyceraldehyde are also shown
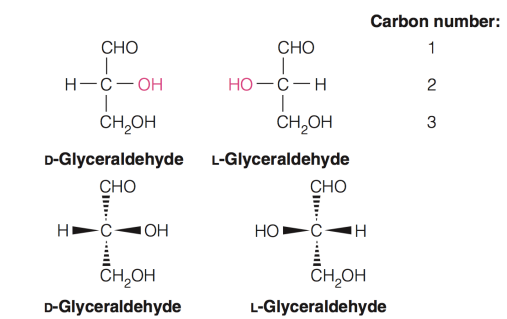
what are enantiomers
Stereoisomers that are mirror images of each other but cannot be superimposed
Stereoisomers that are non-superimposed mirror images of each other are enantiomers
optimal isomer that are mirror images
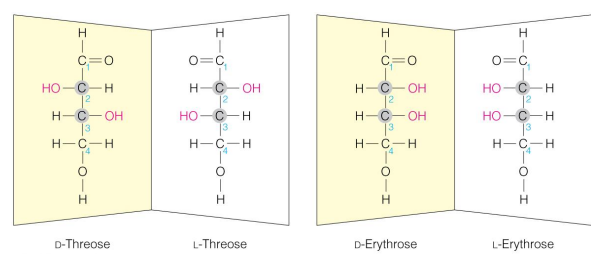
D or L (how it works)
based on the location of the OH on the chiral carbon farther from the carbonyl carbon
D and L isomers of sugars are (relation to each other)
enantiomers
enantiomers have the same
chemical properties but diff physical properties and biological properties
most hexoses in living organisms are D or L
D
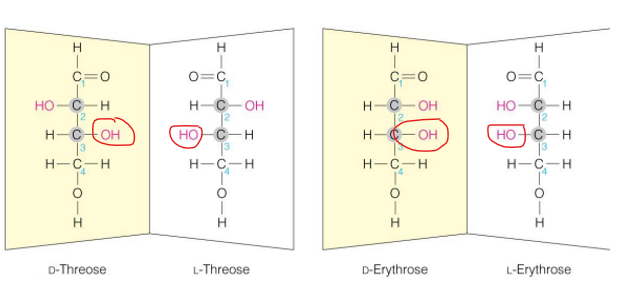
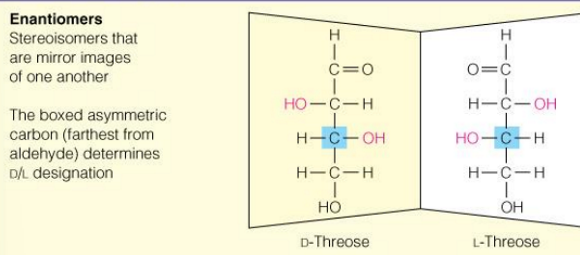
what are Diastereoisomers
Pairs of isomers that have opposite configurations at one or more chiral centers but are NOT mirror images
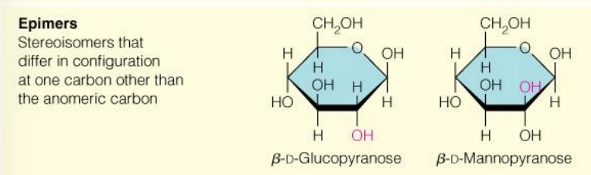
what are epimers
2 sugars that differ in configuration at only one chiral center
what happens to monosaccharides with 5C or more (usually)
form a ring structure in aqueous solution
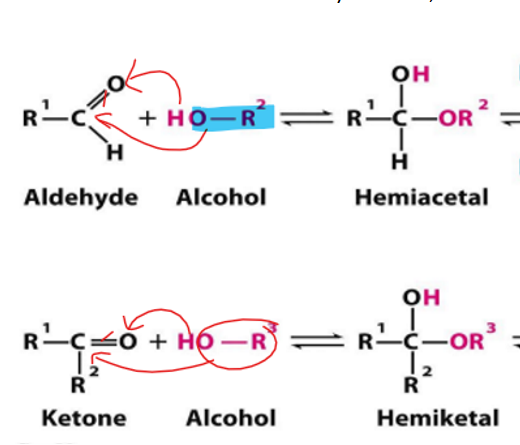
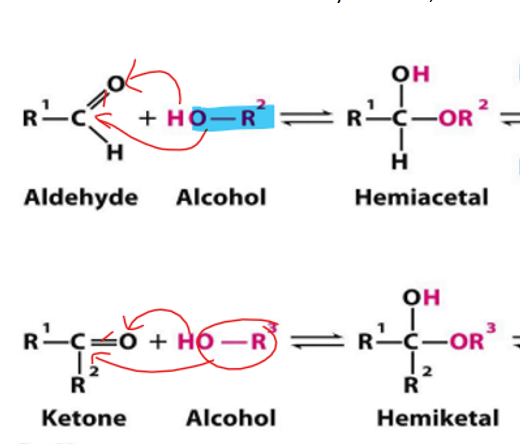
Aldehyde and ketone carbons are
electrophillic
They will accept electrons
They will gain an oxygen (which has a partial negative charge)
alcohol oxygen atom is
nucleophillic
Donating electrons; tend to have a positive or partially positive charge
Gain the hydrogen
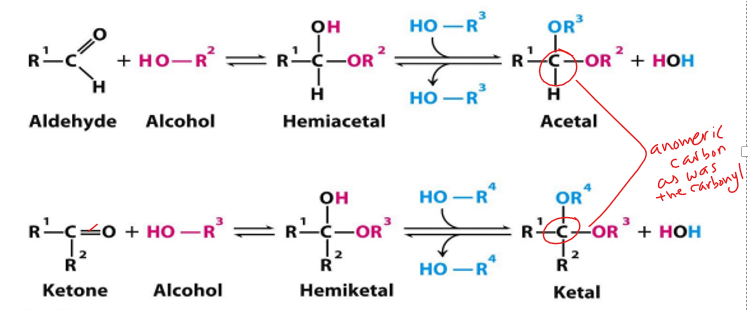
when aldehyde is attacked by alcohols what forms
when ketone is attacked by alcohols what forms
hemiacetal
hemiketal
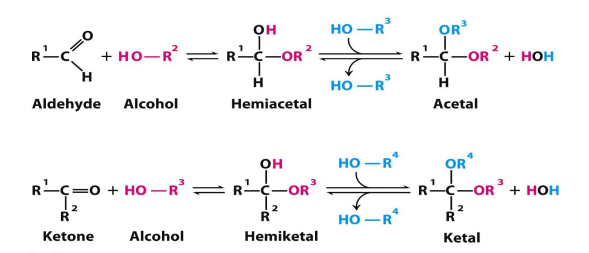
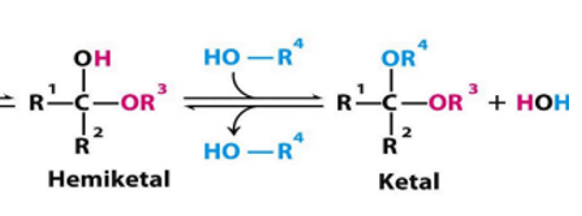
formation of hemiacetel, acetal, hemiketal, ketal
aldehyde or ketone—> hemi (one alcohol added) —> acetal or ketal (2nd alcohol added)
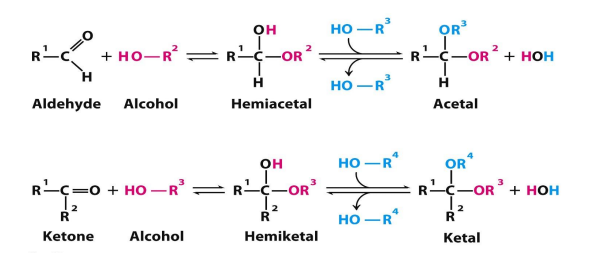
hemiacetal def
when only one alcohol has attacked the aldose
what a single ring sugar is
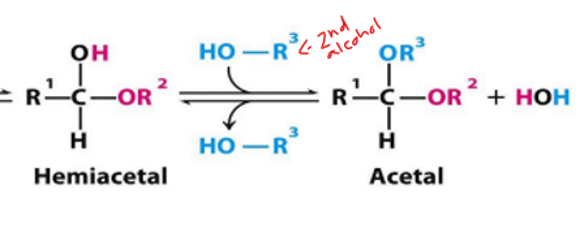
acetal def
when the 2nd alcohol has attacked
hemiacetal—> acetal
what forms when we have dissacharides
hemiketal def
when only one alcohol has attacked the ketose
ketal def
when the 2nd alcohol has attacked
hemiketal—> ketal
glucose is a (functional group)
aldose
glucose can cyclize into a
hemiacetal
fructose is a (functional group)
ketose
fructose can cyclize into a
hemiketal
When hemiacetals and hemiketals are formed, the carbonyl carbon atom becomes a
asymmetric center
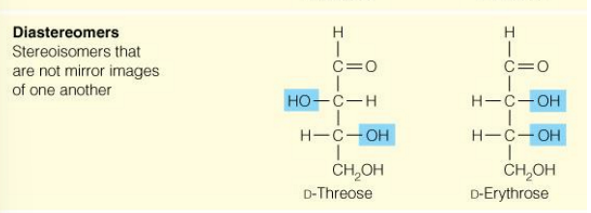
what are anomers
Isomers of monosaccharides that differ only in their configuration about the asymmetric carbon
Cyclic form of glucose is a (anomer name)
pyranose
cyclic form of fructose is a
furanose
what readily undergoes intramolecular cyclization (2)
Pentose and hexoses
when a sugar has undergoes cyclization, what happens to the former carbonyl carbon
becomes a new chiral center
what is it now called
anomeric carbon—> the carbon that was a carbonyl group
what is anomeric carbon
the carbon that was a carbonyl group (C=O) in open structure
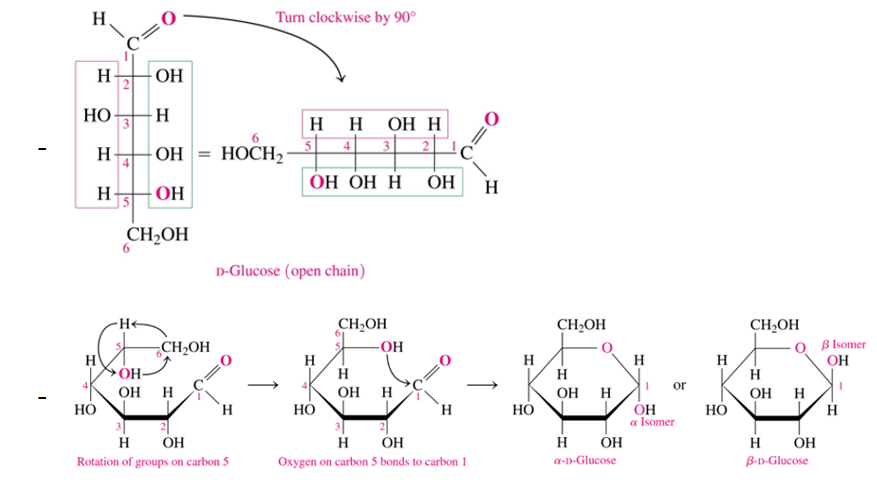
when a sugar has undergoes cyclization, what happens to the former carbonyl oxygen
becomes a hydroxyl group
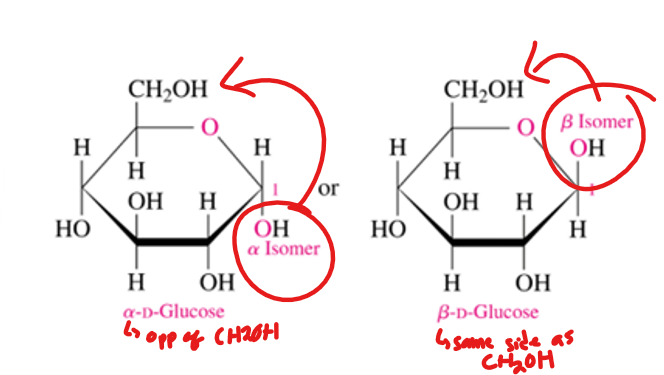
what does the position of the hydroxyl group on annomeric carbon when cyclization has occur determine
if α or β
when is the sugar β or α
alpha when it is on the opposite side (trans) of the ring as the CH2OH
beta when it is on the same side (cis) of the ring as the CH2OH
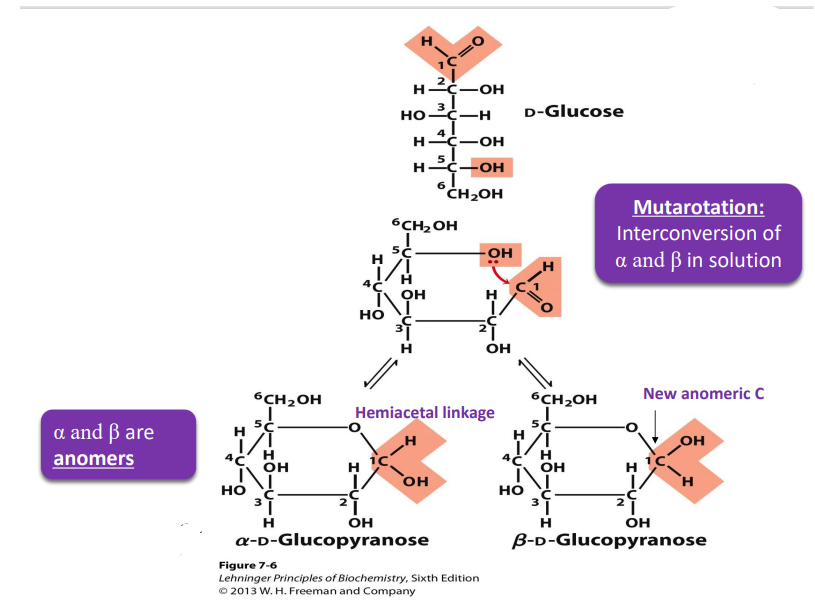
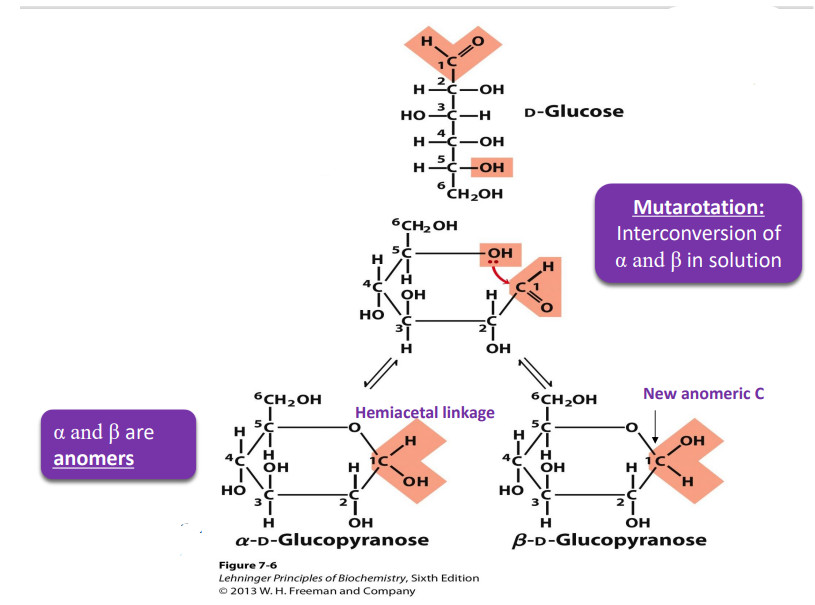
D glucose formation of cyclic form (how, when does it turn beta and when alpha)
If OH is on the left in fisher, it point up in hateworthy
If the OH is on the right, then it points down in Haworth (except for anomeric C)
α and β are anomers
CH2OH is trans to OH on the anomeric C=α
CH2OH is cis to OH on the anomeric C= β
when is a sugar alpha
CH2OH is trans to OH on the anomeric C=α
alpha and beta are
anomers
when is a sugar beta
CH2OH is cis to OH on the anomeric C= β
mutarotation
interconversion of α and β in solution
glucose can undergo (to do with the alpha and beta)
mutarotation
monosaccharides exist in what forms
cyclic, anomeric
what are the 4 most common hexoses
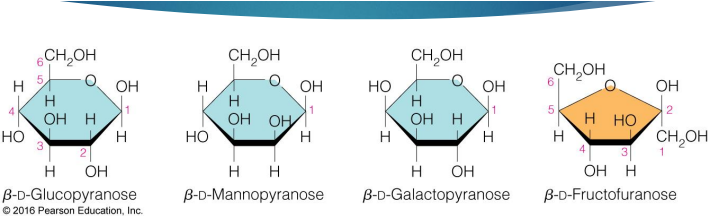
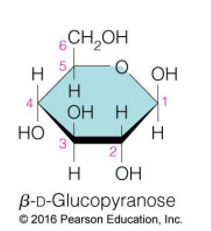
glucose (specific name, structure)
mannose (specific name and structure)
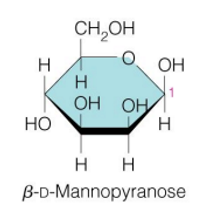
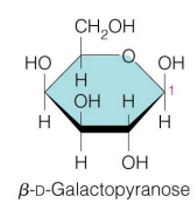
galactose (specific name and structure)
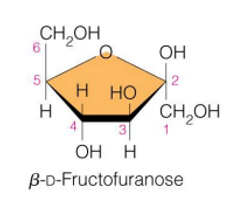
fructose (specific name and structure)
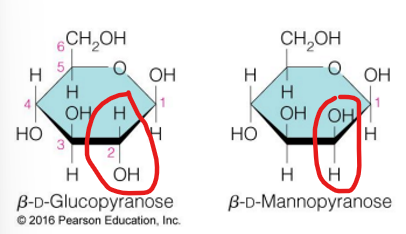
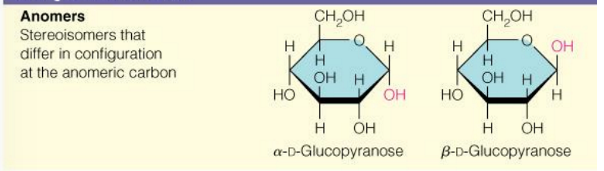
glucose and mannose are
epimers (2 sugars that differ in configuration at only one chiral center)
glucose and galactose are
also epimers
enantiomers
diastereomers
anomers
epimers
monosaccharide derivatives (8)
Sugar acids
Sugar alcohols
Deoxy sugars- ribose and deoxyribose
Sugar esters- like ATP, those with phosphates added
Amino sugars
Acetals, ketals, and glycosides
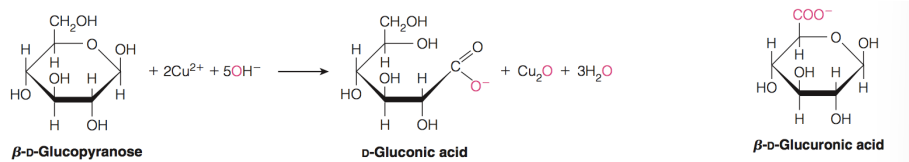
what are reducing sugars
sugars with free anomeric carbons (they can react with other things)
usually C1’
They will reduce oxidizing agents such as peroxide, ferricyanide, and some metals (Cu2+ and Ag+)—> they themselves become oxidized
These redox reactions oxidize the sugar to a sugar acid
is glucose a reducing sugar? used for what?
yes
So these reactions are the basis for diagnostic tests for blood sugar—> HOW IT RELATES TO DIABETES
lactones and acids are produced by
oxidation of sugars (mainly their aldehyde group)
test for reducing sugars
fehlings and tolens
fehlings test
Mild oxidation of aldose with alkaline Cu2+ (fehling's solution) produces aldonic acids as seen in visual "tests"

tolens test
(aldose reduce Ag+ to Ag0

Enzyme-catalyzed oxidation of monosaccharides
gives other products
Uronic acids and glucuronic acid
Oxidation at C6
Important constituents of some polysaccharides
What is mainly used now with the finger prick test
hemoglobin glycation
Hemoglobin becomes irreversibly glycated (Hb A1c)- no enzyme-
Measure of blood glucose control over RBC lifespan (around 3 months) so we get better idea of the glucose levels
Diabetes
When you eat you have glucose in the bloodstream
The glucose will attach to hemoglobin and then go into the RBC
However, diabetics have a higher percentage hemoglobin that has been glycation
HbA1c can eventually form AGE’s (advanced glycated end products)--> not good
Form covalent cross-links with other molecules (proteins)
Can lead to damaged kidneys, retinas, CVD, etc.
so hemoglobin—→ HbA1C—> AGEs
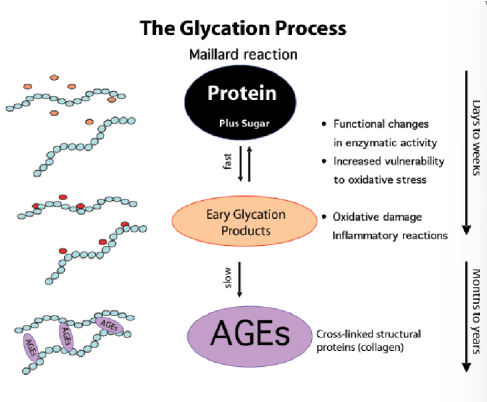
glycation
attaches glucose without an enzyme
glycosylation
attaches glucose using an enzyme
AGEs do what (advanced glycated end products)
Form covalent cross-links with other molecules (proteins)
Can lead to damaged kidneys, retinas, CVD, etc.
deoxy sugars
sugar esters
amino sugars
acetals, ketals, glycosides
Deoxy sugars: constituents of DNA, etc.
Sugar esters: phosphate esters like ATP are important
Amino sugars contain an amino group in place of a hydroxyl group
Acetals, ketals, and glycosides: basis for oligo and polysaccharides
sugar alcohols are what
Sugar alcohols such as sorbitol, mannitol, and xylitol sweeten many “sugarless” gums and candies
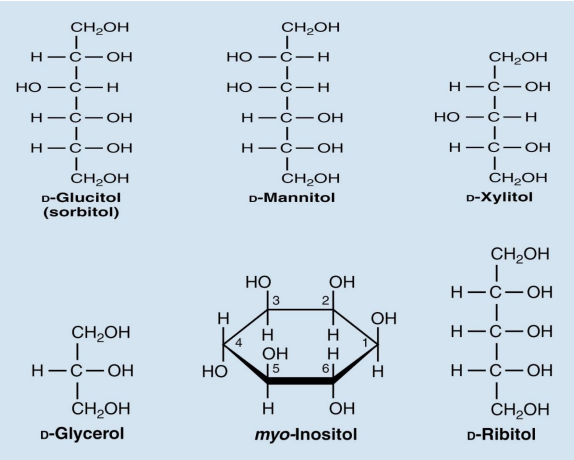
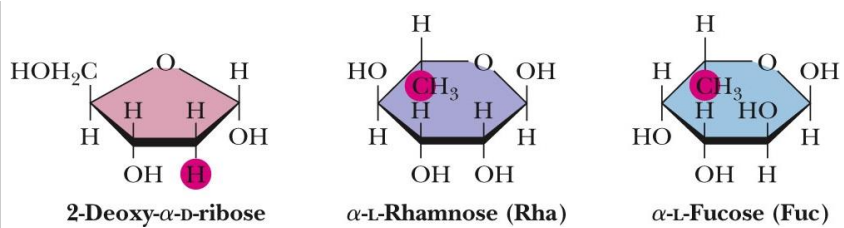
deoxy sugars
Deoxy sugars are monosaccharides with one or more hydroxyl groups replaced by hydrogens
2-Deoxy-D-ribose is a constituent of DNA
Rhamnose is a component of ouabain, a toxic “cardiac glycoside”
Fucose is a component of some cell walls
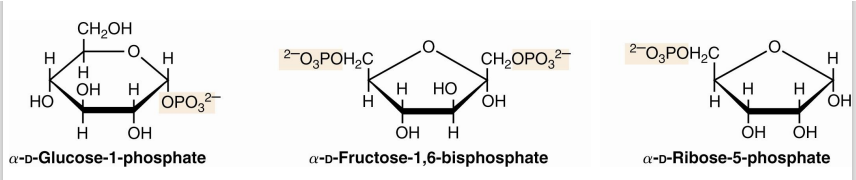
sugar esters
Phosphate esters of glucose, fructose, and other monosaccharides are important metabolic intermediates
They have a phosphates groups added to them
The ribose moiety of nucleotides such as ATP and GTP is phosphorylated at the 5’- position
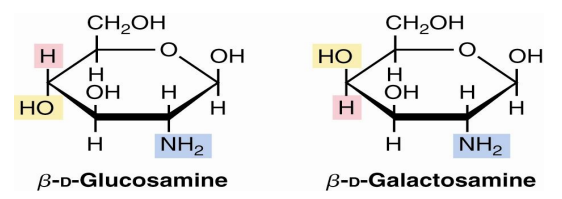
amino sugars
Sugars with an amino group at C-2 are amino sugars.
They are found in many oligosaccharides and polysaccharides.
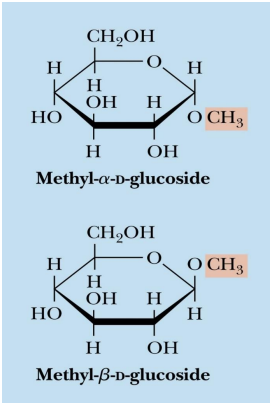
glycosides
The pyranose and furanose forms of monosaccharides react with alcohols in dehydration synthesis reactions to form glycosides, with retention of the α- or β configuration at the C-1 carbon.
The new bond formed is called a glycosidic bond.
In the example, they have a methyl group added to replace the H of the alcohol group
so having a Non sugar group added through dehydration reaction
glycosidic bonds
the linkage between monosaccharides
disaccharides are what
simplest oligosaccharide
linked by glycosidic bonds
each unit in the oligosaccharide is termed as a
residue
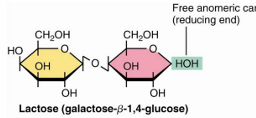
lactose glycosidic bond and residues
galactose and glucose
beta 1→4 glycosidic bond
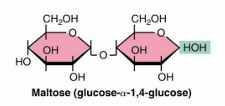
maltose glycosidic bond and residues
2 glucose
alpha 1—>4 glycosidic bon
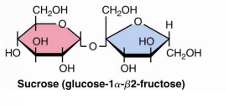
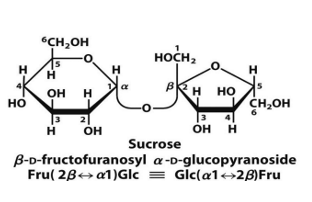
sucrose glycosidic bond and residues
glucose and fructose
alpha, beta 1—> 2 glycosidic bond
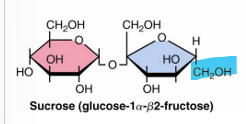
what disaccharide is not a reducing sugar
sucrose
does not have a free anomeric carbon
what is a mixed acetal
2 sugars with one hydroxyl provided intramolecularly and one hydroxyl from the other monosaccharide
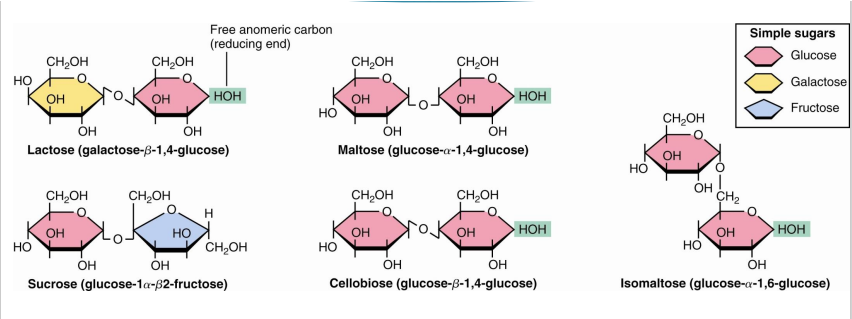
formation of glycosidic bond
2 sugar molecules can be joined covalently via a glycosidic bond
Between an anomeric carbon on the first monosaccharide and a hydroxyl group on the second monosaccharide
Condensation reaction (Water is eliminated)
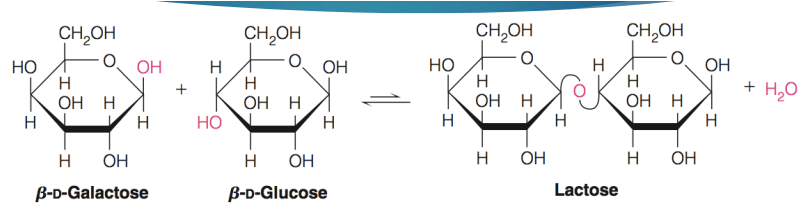
reducing sugar with disaccharides
Anomeric carbon on 2nd monosaccharide still free for additional reactions (reducing sugar)
NOT SUCROSE
glycosidic bond is what type of bond
covalent
reaction that forms glycosidic bond
condensation (water is taken out)
nonreducing disaccharides occur when
Two sugar molecules can be also joined via a glycosidic bond between two anomeric carbons
No free OH group on the anomeric carbon
There are no reducing ends, this is a nonreducing sugar
LIKE SUCROSE
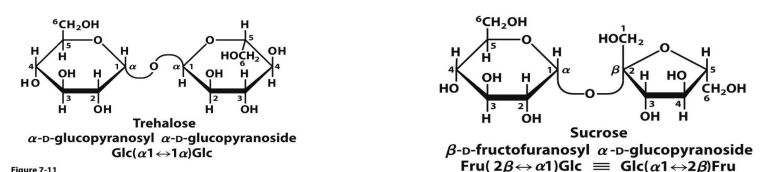
what are the 2 nonreducing disaccharides
trehalose and sucrose
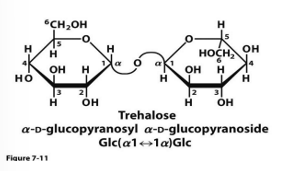
sucrose stability, function (2)
stable toward oxidation and makes it suitable as a storage and transport molecule for energy
because is not easily oxidized and is not reducing sugar
is glycosidic bond reversible
not technically because it is a covalent bond. Would require an enzyme
oligosaccharides are commonly used for
antibiotics
functions of polysaccharides (3)
storage, structure, recognition
homopolysaccharide
a polysaccharide that contains only one kind of monosaccharide
heteropolysaccharide
a polysaccharide made of several monosaccharides
which polys are storage molecules
starch and glycogen