Chapter 7: Alkyl Halides - Nucleophilic Substitution and Elimination Reactions
1/126
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
127 Terms
What is the leveling effect?
(Section 3.7) A base cannot be stronger than the conjugate base of the solvent
In an aqueous solvent (water), hydroxide is the strongest base that can be used
Common way to circumvent this is to use a solvent other than water
Why does the leveling effect matter when looking at the first step of a dehydration reaction’s mechanism?
No acid stronger than hydronium ion (H3O+) can be present in an aqueous environment/solution
What are Alkyl Halides?
Compounds in which a halogen (such as Cl, Br, or I) is connected to an sp3 hybridized carbon atom
What are Aryl and Vinyl Halides?
Compounds in which a halogen is connected to an sp2 hybridized carbon atom
What is an easy way to tell what type of hybridization a carbon atom has?
Look at the number of bonds + number of lone pairs
If there are 4 total bonds / lone pairs, it is sp3
If there are 3 total bonds / lone pairs, it is sp2
Etc.
What two types of reactions do Alkyl Halides typically undergo?
Substitution and Elimination reactions
What happens to the Alkyl Halide in a substitution reaction?
The Alkyl Halide is treated with a nucleophile and the nucleophile ends up replacing the halogen
What happens to the Alkyl Halide in an elimination reaction?
The Alkyl Halide is treated with a base and a pi bond is formed (to create an alkene)
In order to occur, what do substitution and elimination reactions need?
They need a GOOD leaving group to be present on the substrate
Why do substitution and elimination reactions compete with each other?
Many reagents can act as either a nucleophile or base
Example: Hydroxide (—OH)
What term is used to refer to the Alkyl Halide?
Substrate
What two critical functions does the halogen serve in order for the Alkyl Halide to be reactive for S & E reactions?
1. They withdraw electron density via induction, rendering the adjacent carbon atom electrophilic
2. They can serve as a leaving group (which is needed in order for these reactions to occur)
What happens when a carbon atom becomes electrophilic?
It becomes subject to a nucleophilic attack
What is the relationship between acidity and the capability of a leaving group?
A good leaving group is the conjugate base of a strong acid
What is an example of a good leaving group (and why)?
Iodide; I- is the conjugate base of the very strong acid HI
What is an example of a bad leaving group (and why)?
Hydroxide; OH— is not a stabilized base AND it is also a very strong base (meaning that it’s conjugate acid is very weak)
Hydroxide will RARELY ever act as a leaving group
What are some good rules of thumb when judging whether something is a good leaving group?
1. Good leaving groups are typically conjugate bases of strong acids (pKa < o)
2. Chloride, Bromide, and Iodide are all good leaving groups
3. Fluoride is NOT a good leaving group (pKa of HF is 3.2)
4. Iodide is the BEST leaving group of these because it is the weakest base (strongest conjugate acid)
What are the most commonly used leaving groups?
Halides (I-, Cl-, Br-) and Sulfonates (RSO3-)
How are carbon atoms in Alkyl Halides described?
In terms of its proximity to the halogen, using Greek letters
What are the two positions used to describe the carbon atoms in an Alkyl Halide?
Alpha (α) and Beta (β) positions
Alpha — carbon atom directly connected to the halogen
Beta — carbon atoms connected to the alpha position
How many Alpha and Beta positions will an Alkyl Halide have?
1 Alpha position and up to 3 Beta positions
What is an Alpha (α) Position?
Position of a carbon atom directly connected to the halogen of an Alkyl Halide
What is a Beta (β) Position?
Position of a carbon atom connected to the Alpha position of an Alkyl Halide
How are Alkyl Halide classified?
By how many Alkyl Groups are connected to the alpha position
What are the three classifications of Alkyl Halides?
Primary (1˚), Secondary (2˚), and Tertiary (3˚)
Primary - one alkyl group attached to the Alpha position
Secondary - two alkyl groups attached to the Alpha position
Tertiary - three alkyl groups attached to the Alpha position
What are the four steps to naming an Alkane via IUPAC naming classification?
1. Identify and name the parent
2. Identify and name the substituents
3. Number the parent chain and assign a locant to each substituent
4. Assemble the substituents alphabetically
NOTE: The parent chain should be numbered so as for the first substituent to receive the lowest number
You want the parent chain to have the MOST amount of substituents!
When naming Alkyl Halides, how is IUPAC naming different?
Halogens are simply treated as substituents
When a ring contains only on substituent, locants are not used to indicate the location of the substituent
Chiral center configurations must be indicated at the beginning of the name if present in the compound
Common halogenated compound names are recognized by IUPAC
What substituent names are halogens given when named via IUPAC?
(Cl) Chloro-
(Br) Bromo-
(F) Fluoro-
(I) Iodo-
What are the IUPAC rules regarding propyl groups in Alkyl Halides?
Propyl groups are called n-propyl to differentiate them from isopropyl groups
n-propyl and n-butyl are ONLY used in common names
Example: n-propyl bromide
What are some examples of commonly named halogenated compounds?
Iodomethane = Methyl Iodide
2-Chloropropane = Isopropyl Chloride
1-Bromopropane = n-propyl Bromide
Why do systematic names call these compounds haloalkanes?
Because halogens are treated as substituents
What is an Organohalide?
Any organic compound that contains a halogen (including Aryl and Vinyl Halides)
What is the use of most Organohalides?
Insecticides (they are toxic)
What are some examples of some Organohalides used as insecticides?
DDT, Lindane, Chlordane, and Methyl Bromide
DDT - one of the first insecticides used globally
Lindane - used in shampoos to treat head lice
Chlordane and Methyl Bromide - used in fumigants to prevent and treat termite infestations
What are some characteristics of Organohalides?
Particularly stable
Persistent over time (example: DDT)
Regarded as man-made poisons
What is the most abundant Organohalide in the atmosphere?
Methyl Chloride
Why are Organohalides useful?
They can serve as starting materials for the synthesis of more complex molecules
What are the two different types of mechanisms S & E reactions?
Concerted and Stepwise
What is a concerted mechanism?
A mechanism that has events occurring simultaneously
What is a stepwise mechanism?
A mechanism in which events occur separately
How are concerted and stepwise mechanisms the same and different for nucleophilic substitution reactions?
They both involve nucleophilic attack and the loss of a leaving group
However, these events are timed differently
How is rate determined for a nucleophilic substitution reaction?
Based on the concentrations of both the Alkyl Halide and the nucleophile
What order is the reaction process of an SN2 mechanism?
Second-order process in which:
Rate = k [Alkyl Halide] [Nucleophile]
Why is are SN2 and E2 mechanisms said to be bimolecular?
In these mechanisms, there is a step involving TWO chemical entities that collide with one another (the Alkyl Halide and the nucleophile / base)
What is used to refer to bimolecular nucleophilic substitution reactions?
SN2 mechanisms
What type of process is consistent with SN2 reactions?
A concerted process (nucleophile and Alkyl halide are involved in a single step)
During an SN2 reaction, what happens if the alpha position is a chiral center?
There is a change in configuration observed
What configuration change is observed during an SN2 reaction?
Chiral center changes from R (reactant) to S (product)
When a configuration change is observed during an SN2 reaction, what is it said to proceed with?
An inversion of configuration (also called a Walden inversion)
What is the requirement for an inversion of configuration to occur?
Back-side attack; the nucleophile must attack from the back side (opposite side of the leaving group)
What two ways are used to explain why an SN2 reaction proceeds via back-side attack?
1. The lone pairs of the leaving group create regions of high electron density that block the front side of the substrate
2. Molecular Orbital (MO) theory
What type of mechanism is the stereochemical outcome (the inversion of configuration) of an SN2 reaction consistent with?
A concerted mechanism (nucleophile attacks simultaneously with the loss of the leaving group)
How is a transition state represented in a reaction mechanism?
With brackets around it
What does it mean for a reaction to be stereospecific?
The configuration of the reaction product is dependent on the configuration of the starting material
What is the general structure of an SN2 process?
Leaving group is replaced with the nucleophile and the chiral center configuration is inverted
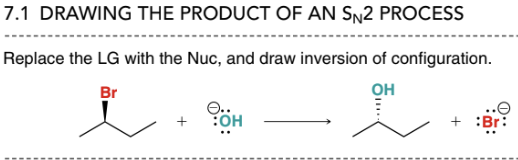
How can the presence of substituents change the rate of an SN2 process?
Putting substituents at alpha or beta positions can significantly reduce the rate of reaction
What two trends can be observed when looking at substituents in SN2 processes?
1. The rate of an SN2 reaction is most sensitive to substituents at the alpha position
2. The rate is also sensitive to the presence of substituents at the beta positions, but this effect is not as great as the effect as the alpha position
These trends occur because of STERIC HINDRANCE on the nucleophile as it approaches the alkyl halide
How is an SN2 process affected if there are three substituents at the alpha or beta position?
The rate is so significantly reduced that the process is too slow to be of any use
What aspects make up the general form of a transition state?
Dotted lines = bonds in the process of being broken or formed
Double-Dagger symbol outside the brackets = indicates the drawing represents a transition state rather than an intermediate
What does a transition state represent?
A maximum (peak) in an energy diagram after the initial activation energy
How does the energy of the transition state affect the rate of the reaction?
Depending on the energy of the transition state, the activation energy will be affected
High transition state energy = high activation energy = slow reaction rate
Low transition state energy = low activation energy = fast reaction rate
What happens to transition state energy when hydrogen atoms are replaced with alkyl groups?
Steric interactions from the alkyl groups will cause transition state energy to become higher
High T.S.E. = high activation energy = low reaction rate
What happens to transition state energy as an alkyl halide becomes more substituted?
The T.S.E. grows higher and higher, causing the activation energy to become higher as well to the point the reaction rate slows
What happens to transition state energy in tertiary alkyl halides?
T.S.E. is so high (as is activation energy) that the reaction rate becomes so slow to the point that the reaction occurs too slowly to be observed
What are the four steps to draw the transition state of an SN2 process?
1. Identify the nucleophile and the leaving group
2. Draw a carbon atom with the nucleophile and leaving group on either side. Use partial charge symbols to indicate partial charges
3. Draw the three groups attached to the carbon atom. Place brackets and the symbol indicating a transition state
What is nucleophilicity?
The rate at which a nucleophile will attack a suitable electrophile
What is the relationship between strength of a nucleophile and its nucleophilicity?
Strong nucleophile = faster SN2 reaction (higher nucleophilicity)
Weak nucleophile = slower SN2 reaction (lower nucleophilicity)
What is generally required in order for an SN2 reaction to be efficient and practical?
A strong nucleophile
What factors can contribute to nucleophilicity?
Presence of charge and polarizability
How does presence of a charge contribute to nucleophilicity?
Negative charges generally indicate higher nucleophilicity (stronger nucleophiles), and vice versa
How does polarizability contribute to nucleophilicity?
Higher polarizability means higher nucleophilicity (stronger nucleophiles), and vice versa
What is polarizability?
Ability of an atom to distribute its electron density unevenly as a result of external influences
What factors affect polarizabilty?
How big an atom is (the size) and the number of electrons distant from the nucleus
Larger atoms will be more polarizable
What are some commonly encountered strong nucleophiles?
Iodide (I-), Bromide (Br-), Chloride (Cl-), HS-, RS-, HO-, RO-, CN- (with a triple bond)
What are some commonly encountered weak nucleophiles?
H2O and ROH (water and alcohols)
Is fluoride a strong or weak nucleophile?
Depends on the solvent; can function as either strong or weak
How are elimination reactions different than substitution reactions?
E reactions involve the use of bases, rather than nucleophiles
How is the strength of an anionic base related to the stability of a base?
Weak bases = stabilized bases
Strong bases = unstabilized bases
What factors affect the stability of an anionic base?
1. Which atom bears the charge
2. Resonance
3. Induction
4. Orbitals
What is the relationship between the strength of a base and its conjugate acid?
Inverse relationship
Strong base = weak conjugate acid
Weak base = strong conjugate acid
What type of reaction do alkyl halides undergo after being treated with a strong base?
Beta elimination reaction (also called 1,2-elimination)
What happens during a beta elimination reaction?
A proton is removed from the beta position
The halide is ejected as a leaving group (X-) from the alpha position
A double bond is formed between the alpha and beta positions
What is another name for when an alkyl halide undergoes a beta elimination reaction?
Dehydrogenation
H and X are removed from the substrate
How is rate determined for a beta elimination reaction?
Based on the concentrations of both the Alkyl Halide and the base
What order is the reaction process of an E2 mechanism?
Second-order process in which:
Rate = k [Alkyl Halide] [Base]
What is used to refer to bimolecular elimination reactions?
E2 reactions
What type of process is consistent with E2 reactions?
A concerted process (base and Alkyl halide are involved in a single step)
What is the difference in rate of reaction for tertiary alkyl halides between SN2 and E2 reactions?
Tertiary alkyl halides react quite rapidly during an E2 reaction, the opposite of what happens with an SN2 reaction
This happens because tertiary substrates have such strong steric hindrance that the reagent can NEVER effectively act as a nucleophile, but it can function effectively as a base
What is the difference between SN2 and E2 reactions in the role played by the reagent?
SN2 —> reagent acts as a nucleophile and ATTACKS the alpha position
E2 —> reagent acts as a base and ABSTRACTS (removes) a proton from a beta position
What is the relative stability of cis alkenes to its stereoisomeric trans alkenes?
Cis alkenes are generally LESS stable than their stereoisomeric trans alkenes
Why are cis alkenes generally less stable than their trans alkenes?
Steric strain in the cis isomer causes the instability
How can you compare energy between cis and trans stereoisomeric alkenes?
By comparing their heat of combustions;
The alkene with the higher absolute value of heat of combustion will be more stable
How does degree of substitution affect stability of alkenes?
Alkenes are more stable when they are highly substituted
What is hyperconjugation?
A stabilizing effect that enables delocalization of electron density
When is a cis configuration inferred with cycloalkenes?
When there are seven or fewer carbon atoms in the ring
What is Bredt’s Rule?
A double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough (8 carbon atoms)
How many products are produced in elimination reactions?
They can produce more than one possible product, but some only produce one
What is regiochemistry?
Where a change takes place in a reaction
What is said to happen when a reaction produces more than one possible product?
The reaction is said to produce two (or more) regiochemical outcomes
In the case of there being more than one product, what is the relative amount formed for each product?
One of the alkene products will be produced more (major product), while the other is produced less (minor product)
Generally, the major product will be the more substituted alkene