AP Chemistry Unit 1, 2, and 3
1/38
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
1 mole
A unit in chemistry that represents 6.022 x 10²³ particles, atoms, or molecules of a substance. It is used to convert between the mass of a substance and the number of particles it contains.
PV=NRT
An equation representing the ideal gas law, where P is pressure, V is volume, N is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin.
Standard Conditions
Standard conditions refer to a specific set of temperature and pressure for measuring gases, typically defined as 0 degrees Celsius (273.15 K) and 1 atmosphere of pressure.
Empirical Formula Steps
An empirical formula represents the simplest integer ratio of elements in a compound. The steps to determine it involve calculating the moles of each element in a sample, dividing by the smallest number of moles, and expressing the ratio as whole numbers.
Coulomb’s law
The equation that describes the force between two charged particles, defined as F = k(q1*q2)/r², where F is the force, k is Coulomb's constant, q1 and q2 are the charges, and r is the distance between the charges.
Excited state
A higher energy state of an atom or molecule, where one or more electrons have absorbed energy and jumped to a higher energy level, differing from the ground state.
Anion
A negatively charged ion formed when an atom gains one or more electrons. (Are bigger)
Cation
A positively charged ion formed when an atom loses one or more electrons. (Are smaller)
Electron Shielding
The phenomenon where inner shell electrons reduce the effective nuclear charge felt by outer shell electrons, affecting their energy levels and chemical behavior.
Metallic Bonding
A type of chemical bond that occurs between metal atoms, characterized by the sharing of free electrons among a lattice of cations, allowing for conductivity and malleability.
Substitutional Alloy
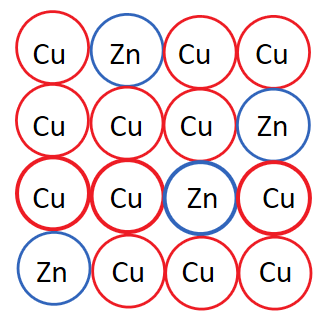
Interstitial Alloy

Sigma Bond
Single Bonds
Pi Bond
Double Bonds
Covalent Network
A type of chemical bond where atoms are connected by covalent bonds in a continuous network, resulting in strong materials with high melting points.
Ionic Conductivity
The ability of a substance to conduct electricity through the movement of ions in a solution or molten state. Ionic compounds are typically poor conductors in solid form but can conduct electricity when dissolved in water or melted.
Formal Charge
The charge an atom would have in a molecule if all bonding electrons were shared equally, calculated by the formula: Formal Charge = Valence Electrons - (Nonbonding Electrons + 1/2 Bonding Electrons). It helps in evaluating the most stable Lewis structure.
Bond Angles
180, 120, 109, 90
2
Linear
3
Trigonal Planar, Bent
4
Tetrahedral, Trigonal Pyramidal, Bent
5
Trigonal Bipyramidal, Seesaw, T-shaped, Linear
6
Octahedral, Square Pyramidal, Square Planar, T-shaped, linear
Intermolecular Forces
Forces that occur between molecules, affecting their physical properties like boiling and melting points. They include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. These must be broken for a phase change to occur.
Dipole-Ion
interactions occur between a dipole and an ion, important in solutions where ionic compounds are dissolved in polar solvents.
Dipole-Induced
interactions occur when a polar molecule induces a dipole in a nonpolar molecule, affecting the overall behavior and stability of the system.
Dipole-Dipole
interactions occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another, significantly influencing the boiling and melting points of substances.
IMF Strength
The order of intermolecular forces by strength is hydrogen bonds, dipole-dipole interactions, and dispersion forces. This ranking helps predict physical properties such as boiling and melting points.
Vapor Pressure
is the pressure exerted by a vapor in equilibrium with its liquid or solid form at a given temperature. It is an important property that influences phase changes and volatility. Opposite to boiling point
Like Dissolves Like
is a rule in chemistry stating that polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes, which relates to the concept of intermolecular forces.
Chromotography
is a technique used to separate and analyze mixtures based on the differential affinities of compounds for a stationary phase and a mobile phase.
Renention Factor
is a ratio used in chromatography to describe the relative distance traveled by a compound compared to the solvent front. It helps identify and quantify substances in a mixture.
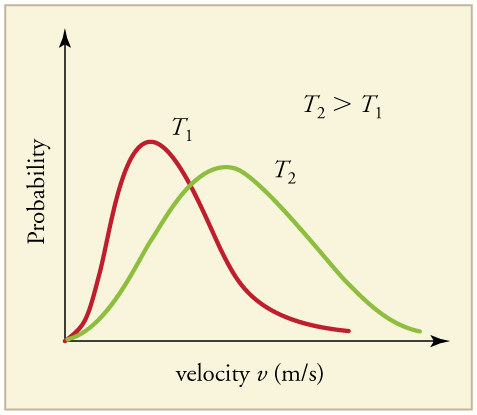
Maxwell-Boltzman Graph
is a graphical representation that shows the distribution of speeds of particles in a gas at a given temperature, illustrating how temperature affects molecular motion and the energy distribution.
Effusion
is the process by which gas particles escape from a container through a small hole into a vacuum, influenced by the speed of the gas particles and their kinetic energy.
Effusion Rate
describes the rate at which gas particles escape through a small opening. If the gas is heavier it will have a lower rate. If it is at a lower temperature it will have a lower rate.
Partial Pressure
is calculated using Dalton's Law of Partial Pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas present. The partial pressure of a gas can be found using the formula: Ppartial = (ngas / ntotal) * Ptotal, where n is the number of moles of the gas and P_total is the total pressure.
Deviations from ideal gas law
occur when real gases exhibit behaviors that differ from the predictions of the ideal gas law, often due to interactions between gas particles or at high pressures and low temperatures. The volume of a gas becomes significant at high temperatures.
Density
mass/volume, higher density sinks
Meter to Nanometer
1 m = 1 x 10^9 nm