Molecule shapes
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Trigonal planar
Has 3 bonded electron pairs around the central atom
Has a bond angle of 120 degrees around the central atom

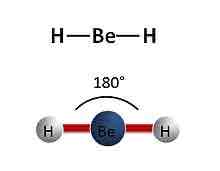
Linear molecule
Has 2 bonded electron pairs around the central atom
Has a bond angle of 180 degrees around the central atom
Tetrahedral
Has 4 bonded electron pairs around the central atom
Has a bond angle of 109.5 degrees around the central atom\
Has one molecule sticking towards you and one away from you

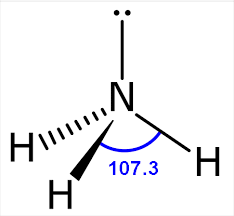
Triagnol pyramid
Has 3 bonded electron pairs around central atom and one lone pair
Has a bond angle of 107 degrees around atom
Has one molecule sticking away from you and one towards you
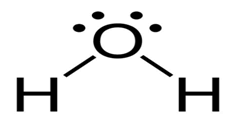
Non-linear
Has a 2 bonded electron pairs around central atom and 2 lone pairs
Has a bond angle of 104.5 around the central atom
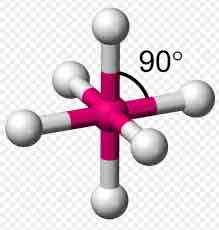
Octahedral
Has 6 bonded electrons around central atom
Has a bond angle around the central atom of 90 degrees
Has two molecules sticking away from you and two molecules sticking towards you
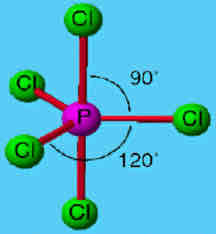
Trigonal bipyramidal
Has 5 bonded electron pairs around central atom
Has a bond angle of 90 degrees and 120 degrees around central atom
Has one molecule sticking away from you
Has one molecule sticking towards you
Solid wedge
Bind pointing towards you
Broken lines
Bond sticking away from you