Organic Chemistry Exam 1
1/62
Earn XP
Description and Tags
Functional groups, pKa values, terms to help name compounds, etc.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms

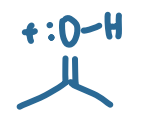
pKa value?
-9
pKa value?
-7.3

pKa value?
-7
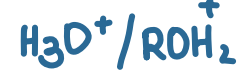
pKa value?
-1.7
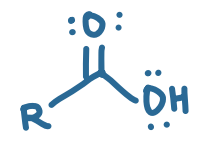
pKa value?
4.75
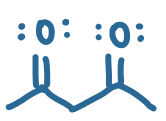
pKa value?
9
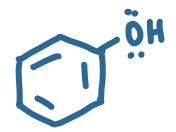
pKa value?
~10

pKa value?
10
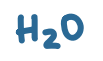
pKa value?
15.7

pKa value?
~16
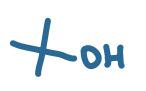
pKa value?
18

pKa value?
19.2

pKa value?
25

pKa value?
36
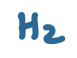
pKa value?
36
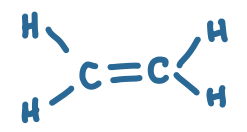
pKa value?
44

pKa value?
49

pKa value?
50
The stronger the acid the more ___________ (positive/negative) the pKa value gets.
Negative
Acid
Proton (H+) donor, A in acid for AWAY
Base
Proton (H+) acceptor
Determining a base
Negative charge, metal ion
Determining an acid
Positive charge
Determining equilibrium arrows
Compare pKa values of acid and conjugate acid. Arrow goes toward higher pKa value (weaker acid)

What functional group is this?
Alkane

What functional group is this?
Alkene

What functional group is this?
Alkyne
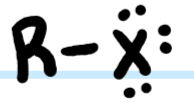
What functional group is this?
Alkyl Halide

What functional group is this?
Alcohol

What functional group is this?
Ether

What functional group is this?
Thiol

What functional group is this?
Thioether
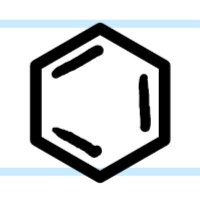
What functional group is this?
Benzene/Aromatic

What functional group is this?
Amene
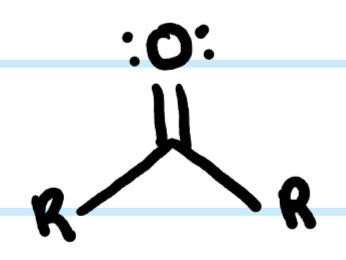
What functional group is this?
Ketone
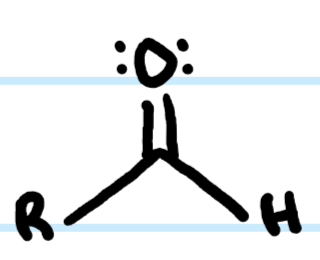
What functional group is this?
Aldehyde
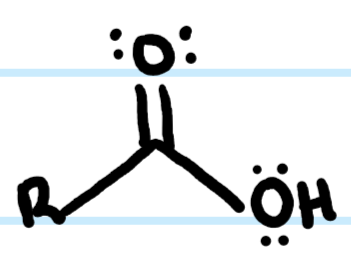
What functional group is this?
Carboxylic Acid
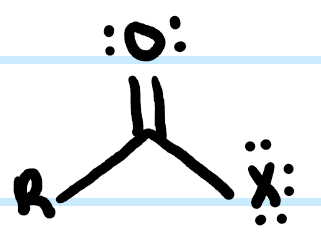
What functional group is this?
Acid Halide
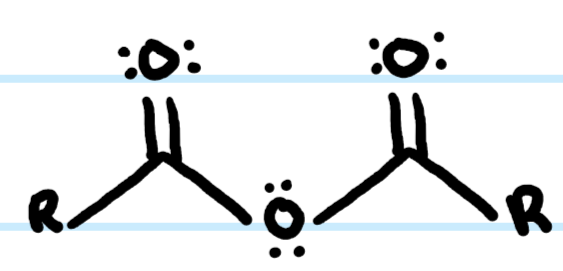
What functional group is this?
Anhydride
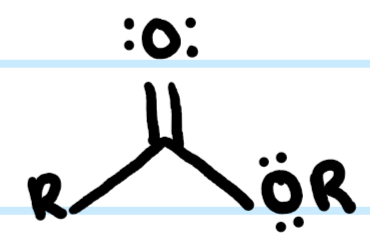
What functional group is this?
Ester
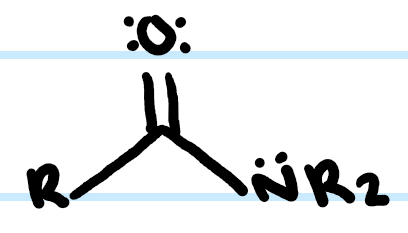
What functional group is this?
Amide

What functional group is this?
Nitrile
sp³ hybridized
atom is connected to 4 things
sp² hybridized
atom is connected to three things
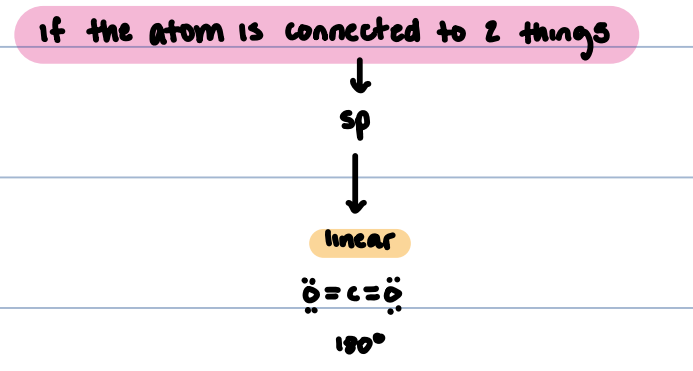
sp hybridized
atom is connected to one thing
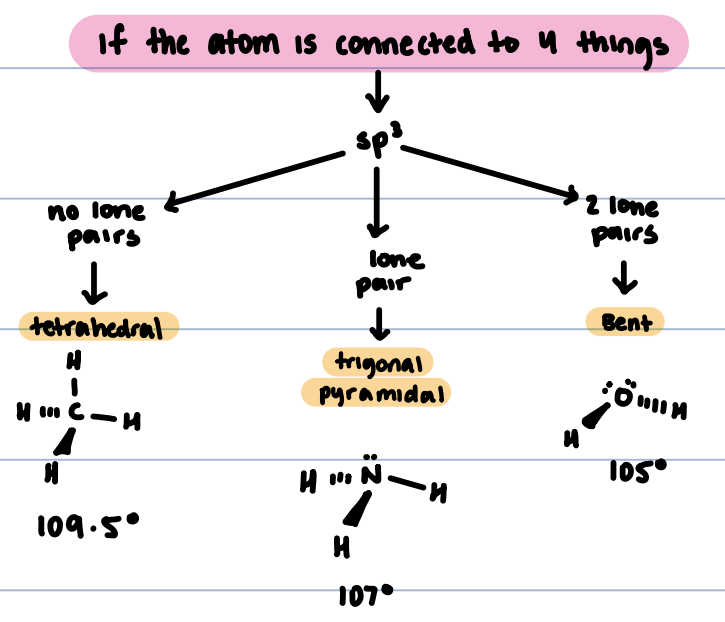
sp³ hybridized structures
No lone pairs: tetrahedral, 109.5°
One long pair: trigonal pyramidal, 107°
Two lone pairs: Bent, 105°
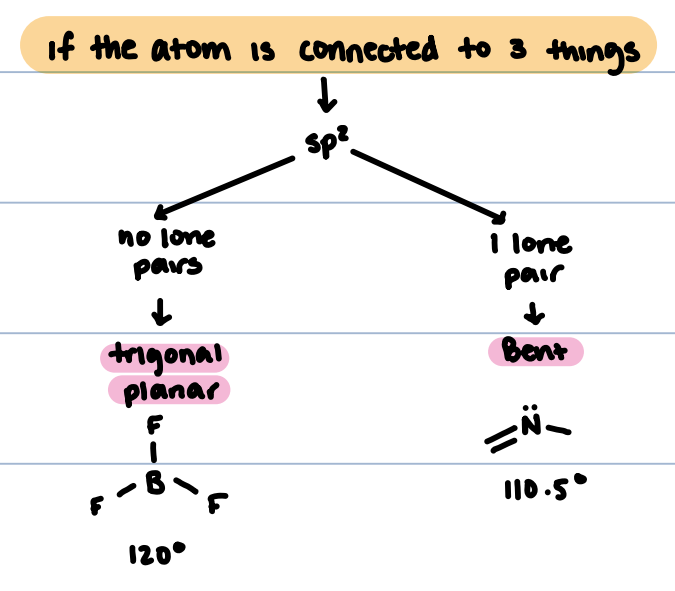
sp² hybridized structures
No lone pairs: trigonal planar, 120°
One lone pair: bent, 110.5°
sp hybridized structure
Linear, 180°
What is a dipole moment?
Uneven sharing of electrons
Formal charge equation
valence electrons - # of dots - # of lines
valence electrons - # of lone pairs - # of bonds
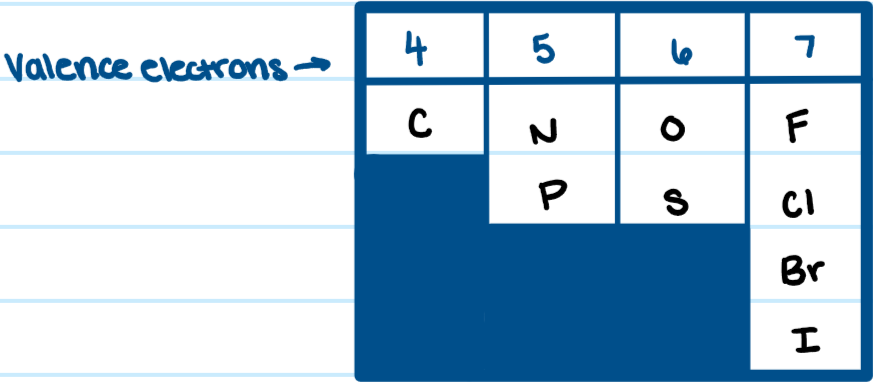
Valence Electrons of important elements
How many carbons does methane have?
1
How many carbons does ethane have?
2
How many carbons does propane have?
3
How many carbons does butane have?
4
How many carbons does pentane have?
5
How many carbons does hexane have?
6
How many carbons does heptane?
7
How many carbons does octane have?
8
How many carbons does nonane have?
9
How many carbons does decane have?
10
How many carbons does undecane have?
11
How many carbons does dodecane have?
12