Chemistry - Atomic Spectra
1/12
Earn XP
Description and Tags
Electromagnetic Radiation, Emission Spectrum, Flame Emission Spectroscopy, Absorption Spectrum, Atomic Absorption Spectroscopy
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Electromagnetic Radiation
-Visible light is a form of electromagnetic radiation.
Electromagnetic spectrum
-Range of different forms of electromagnetic radiation
-Visible light is a small part of the electromagnetic spectrum
Bohr’s theory
-He determined that electrons possess a specific amount of energy dependent on the shell they occupy
-He suggested that electrons could move between the energy levels by absorbing or emitting energy in the form of light
Quantised energy
-The amount of energy that is possessed by an electron in a specific shell - different for all elements
Excited state
-Electrons gain energy when heated and become excited.
-Causes them to move into a higher energy level/electron shell
-Unstable state
Ground state
-When electrons release the absorbed energy as light
-The light released has a specific value which correlates to a specific wavelength of visible light (colour)
Emission Spectrum
-Light released when an element is given energy and the electrons release that energy as light
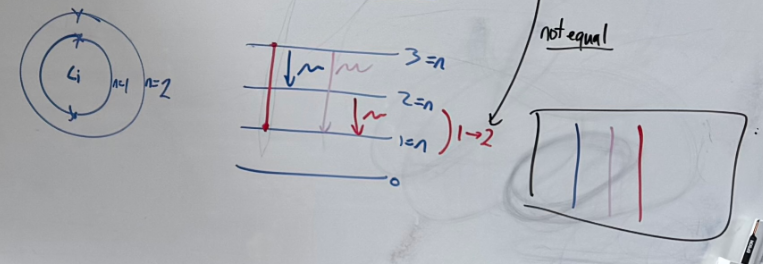
How emission spectra are evidence for electron shells in Bohr model
Each line in an emission spectrum corresponds to a specific amount of energy
-The energy is emitted when electrons from higher energy shells move to lower
-Different lines indicate that there are differences in energy between shells.
Flame Emission Spectroscopy (Flame Photometry)
-A sample is atomized in a flame, exciting metal ions to emit light at specific wavelengths.
-The light intensity indicates the concentration of the metal in the sample
Absorption Spectrum
-Electrons can enter the ‘excited state’ if they are able to absorb the exact amount of energy needed to move to a higher energy level/electron shell
-Results in specific wavelengths of light being absorbed from the light space.
-This leaves a black visible line to correspond to a light spectrum
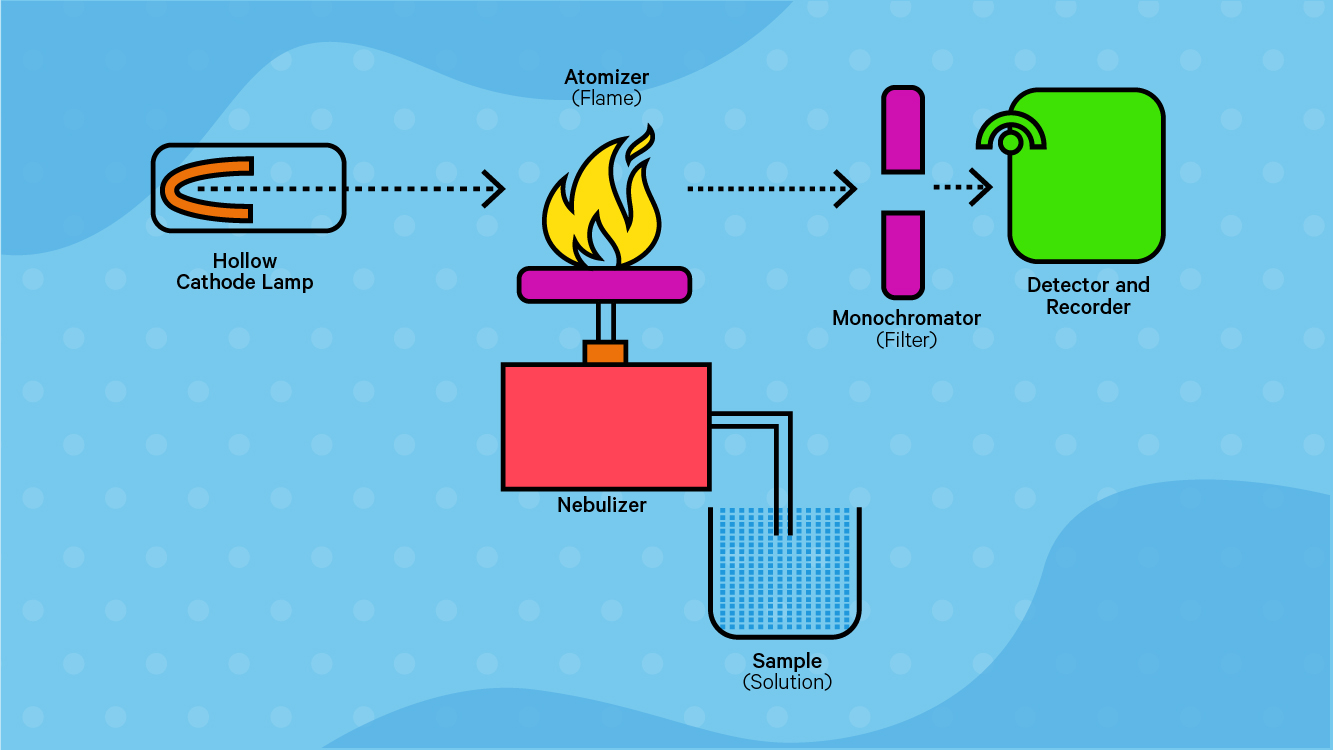
Atomic Absorption Spectroscopy (AAS)
1.Nebuliser converts sample solution into fine mist so that is it quickly heated and vaporised by the flame
2.Flame vaporises sample and breaks apart most molecules into individual atoms
3.Hollow Cathode lamp: produces wavelengths of light which correspond to the emission spectrum of metal - detect only metal being analysed
4.Monochromator isolates a single wavelength of light to be analysed to reduce interference
5.detector measures intensity of light absorbed by sample - determines concentration of metal being analysed
AAS measures:
-AAS records the absorbance of light, a unitless (dimensionless) measurement.
-Using a calibration curve, concentrations are typically reported in mg/L, ppm, or ppb
AAS is only useful for analysing metals. Why?
-The hollow cathode lamp needs to be made using the element being analysed.
-Because non-metals generally do not conduct electricity, a hollow cathode lamp cannot be produced using a non-metal.