Isomerism
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Two main types of isomerism
Structual
Stereo
Structural Isomerism
When substances have the same molecular formula but different structural formula
Different types of Structural Isomerism
chain
position
functional group
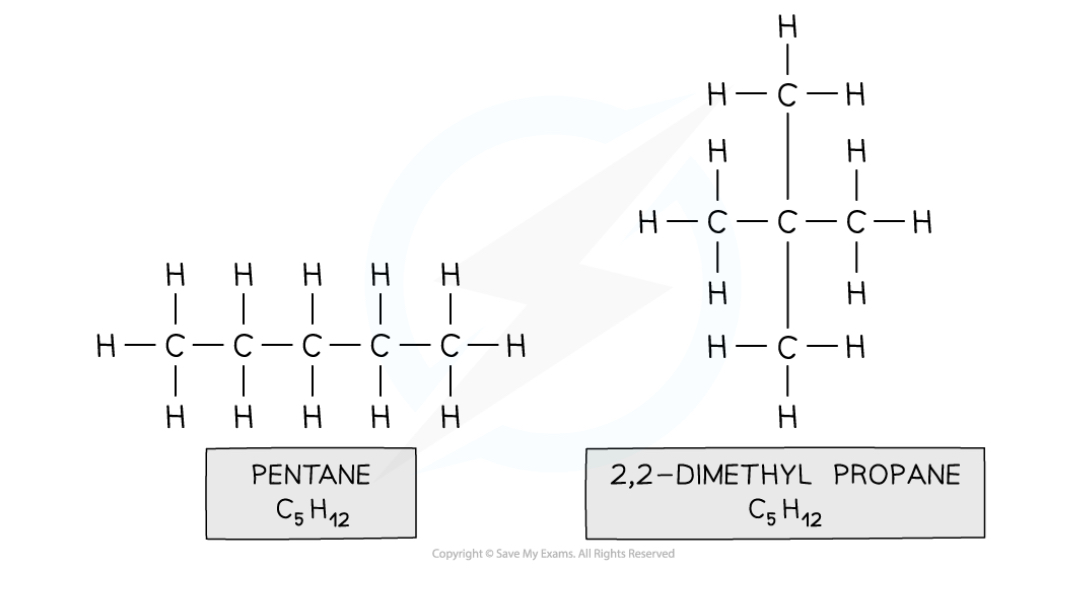
Chain Isomers
when compounds have the same molecular formula, but their longest hydrocarbon chain is not the same
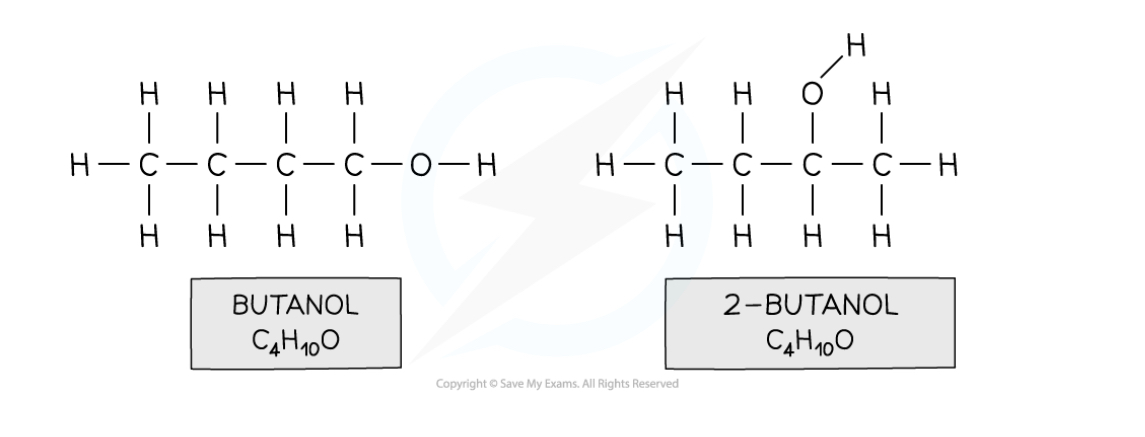
Position Isomers
When substances have their functional groups in different positions
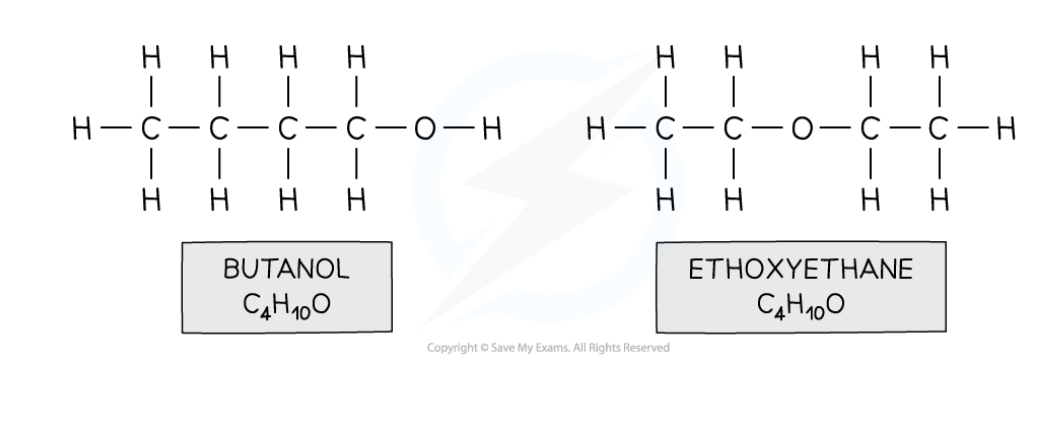
Functional group isomers
They have the same molecular formula but have different functional groups.
Steroisomerism
substances have the same molecular formula and structural formula but have a different spatial arrangement of atoms
Types of Steroisomerism
E/Z isomerism
Z form
when two atoms of each pair of higher atomic number are on the same side of the carbon double bond (top or bottom)
E form
when two atoms of each pair of higher atomic number are on the opposite side of the carbon double bond (top or bottom)
How is priority group decided?
It has a higher atomic number