SR10-Thermochemical Processing
1/14
Earn XP
Description and Tags
LO: 1. Understand the nature of redox reactions 2. Know means to chemically reduce minerals to produce oxygen 3. Be able to evaluate the different processes associated thermochemical reduction
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Define what Redox-Reaction is in terms of electrons ?
Oxidation: Loss of electrons; increase of charge
Reduction: Gain of electrons; reduction of charge
Redox Reaction: Reduction and Oxidation occur simultaneously (one subtance reduces, the other oxidises)
Example: Combustion/Oxidation of Carbon: C + O_2 —> CO_2
C donates 2 electrons to each O to fill the valence shell (complete the octet on the valence shell) —> Oxygen is an oxidising agent (gains electrons)
Remeber:
In ISRU applications, reduction of metal oxides yields metal and oxygen
These reactions are redox reactions based on the transfer of electrons
To enable these reactions, we need reducing agents (electron donors) and temperature (activation)
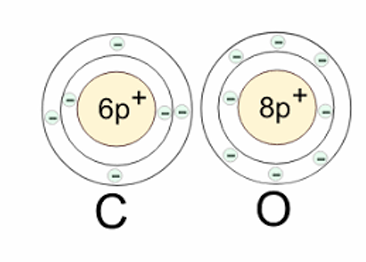
What is the correct result for the oxidisation of a metal?
a) M + O⟶M^+2 + O^-2
b) M + O⟶M^-2+ O^+2
c) M + O⟶M^+2 + O^+2
What are the main practical challenges for solid-gas interaction?
Main challenges are:
Homogeneous heat distribution
Solid-gas exposure
Energy requirements
Solution: Adoption of terrestrial industry processes
- Rotary dryer
- Rotary kiln
- Fluidised bed
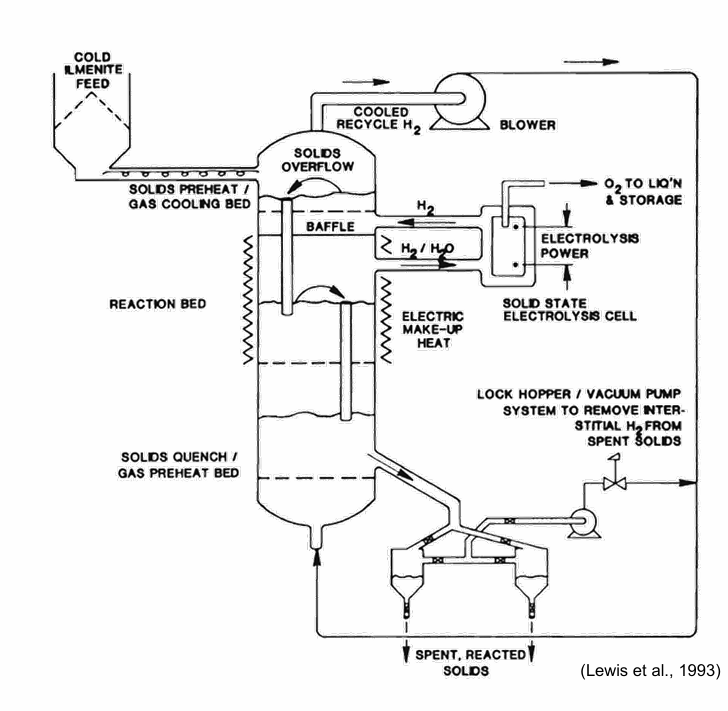
How is the mineral Ilmenite reduced with H_2? What is its redox reaction ?
Reduction: FeTiO3 + H2 ⟺ Fe + TiO2 + H2O
Electrolysis: H2O ⟺ H2 + ½ O2
Ilmenite is reduced at 700–1000 °C in the presence of hydrogen
Equilibrium reaction, mildly endothermic (∆H = 9.7 kcal/(g·mol) at 900 °C)
Mole fraction of water needs to remain <10% to maintain the reaction
Ilmenite is most susceptible to reduction of iron (next comes glass, then olivine, then pyroxene)
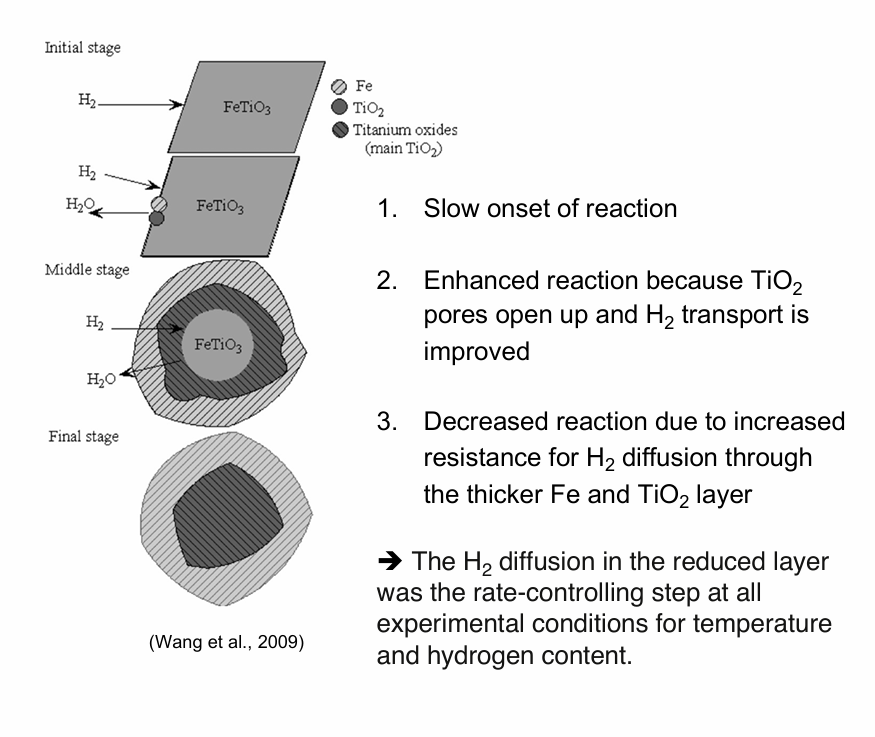
How does the shrinking core process/shrinking core model of illeminite look like (in steps)?
Diffusion of the reactant through a product TiO2 layer
Reaction with the ileminite core
Diffusion of iron out of the TiO2 pores
Formation of metallic iron outside the ileminite grains
What is the goal of the Illeminite reduction process proposed and patented by Carbotek Inc. in 1989 ?
→ Lunar Liquid Oxygen (LLOX) production by fluidised bed reactor
How is the Glass reduced with H_2? What is its redox reaction ?
Reduction: FeO(glass) + H2 ⟺ Fe + H2O
Electrolysis: H2O ⟺ H2 + ½ O2
Glass produced by impacts (agglutinates) or volcanic activity contains up to 20 wt% FeO
Glass has faster reaction kinetics compared to silicates
Glass found in pyroclastic deposits is comparably pure (less beneficiation required), but not as abundant across the lunar surface
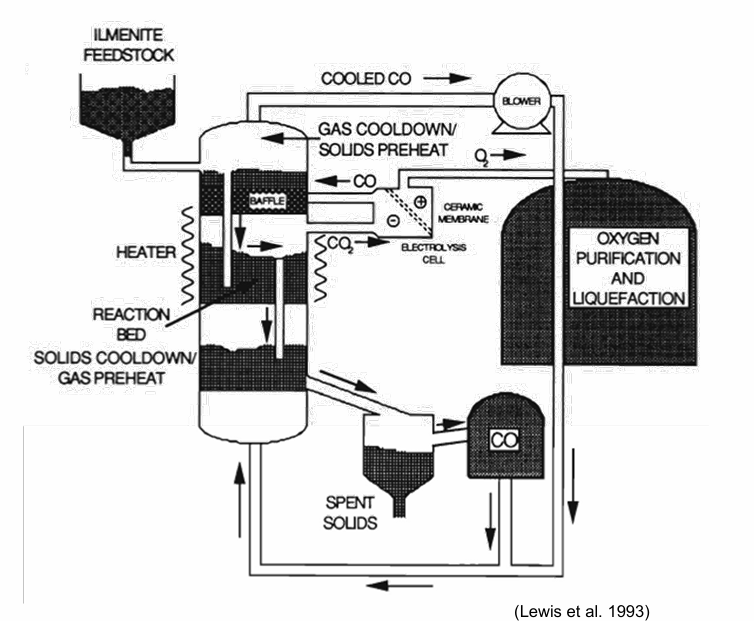
How is the Illeminite reduced with CO? What is its redox reaction ?
Reduction: FeTiO3 + CO ⟺ Fe + TiO2 + CO
CO2 cracking: CO2 ⟺CO + ½ O2
Similar process as for reduction with hydrogen, but with slower reaction rate
Activation energy using CO is 29.6 kcal/mol as compared to 22.3 kcal/mol using H2
Similar to hydrogen, the reduction follows the shrinking core model
CO2 cracking, e.g. via a plasma, is a highly endothermic reaction
Solar wind implanted carbon could be used to replenish the reactant (but only several tens of ppm)
How is the Illeminite reduced with CH4? What is its redox reaction ?
Reduction: FeTiO3 + CH4 ⟺ Fe + TiO2 + CO + 2 H2
Hydrogenation: CO + 3 H2⟺CH4+ H2O
Electrolysis: H2O ⟺ H2 + ½ O2
Similar process as for reduction with hydrogen
Reaction temperature starts around 1000 °C and involves decomposition of CH4 into C and H2 (the respective reaction with H2 might apply depending on conditions)
Solar wind implanted hydrogen and carbon could be used to replenish the reactan
What are the two different reduction variants in Carbothermal reduction? What is its redox reaction ?
Reduction (variant 1): Mg2SiO4 + 2 CH4 ⟺ 2 MgO + Si + 4 H2 + 2 CO (here: olivine and methane)
Reduction (variant 2): FeTiO3 + C ⟺ Fe + TiO2 + CO (here: ilmenite and carbon)
Hydrogenation: CO + 3 H2⟺CH4+ H2O
Electrolysis: H2O ⟺ H2 + ½ O2
No beneficiation required, different silicates and oxides are reduced in the process
Carbothermal includes all processes involving molten reactant and carbon in some form
As CH4 decomposes into the two reactants C and H2 prior to the reaction, C/CO/CO2 can also be used
For highest efficiency and reduction of silicates this process requires a melt (1600–2000 °C)
Solar wind implanted hydrogen and carbon could be used to replenish the reactant
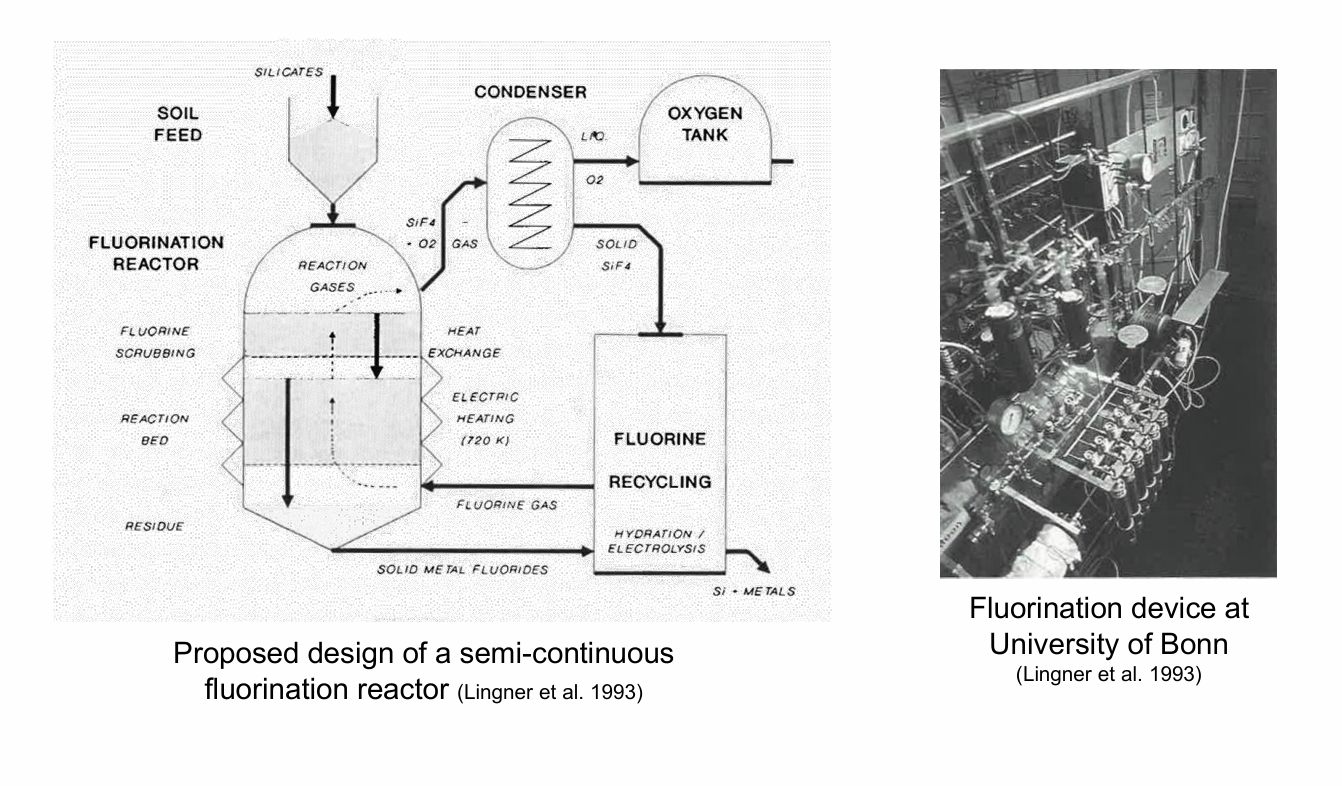
How does the extraction process with flourine look like?
Reduction (general): MxyOy + y F2 ⟶ xy MF2/x + y/2 O2
Reduction (Aluminium oxide): Al2O3 + 3 F2 ⟶ 2 AlF3 + 3/2 O2
Reduction (Olivine): (Fe, Mg)2SiO4 + 4 F2 ⟶ 2 (FeF2, MgF2) + SiF4 + 2 O
Fluorine is highly electronegative (strong oxidiser), about 80% of the total oxygen can be released (potentially 100% for olivine-free highland soil)
Moderate temperatures required (350–650 °C)
Silicates combine with fluorine to build fluorides, and oxygen is liberated
This is a common process for (lunar) rock analysis and first demonstrated in 1962
Fluorine is toxic to humans
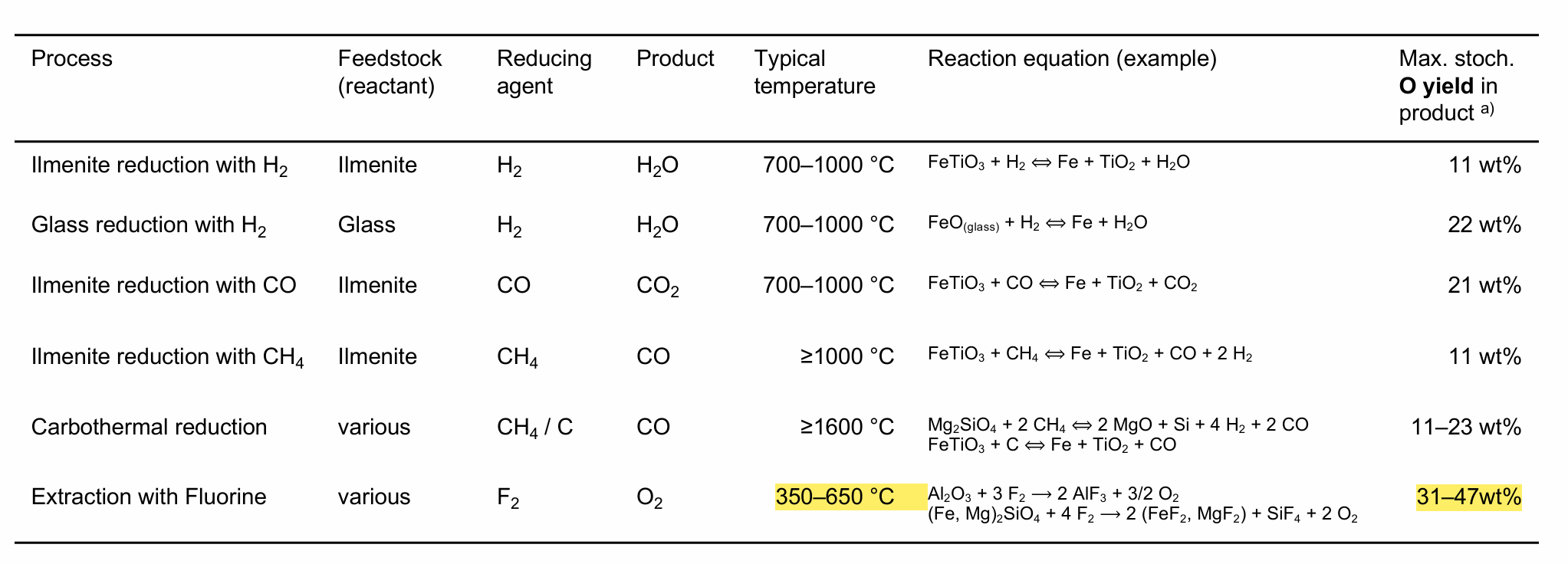
Which solid-gas interaction process yields the most oxygen/O2 in wt%
What special process is used on the ISS to support the recovery of oxygen ?
→ Sabatier Process
Reaction: CO2 + 4 H2 ⟺CH4 + 2 H2O
Electrolysis: H2O ⟺ H2 + ½ O2
Atmospheric CO2 (96% on Mars) is converted to methane and water at 300–400 °C and ~30 bar
catalyst: Nickel or ideally ruthenium on alumina
Water is electrolysed and the residual hydrogen is fed back into the first reaction
The process is used on the ISS to support the recovery of oxygen
CH4 and O2 can be used as fuel (combustion: CH4 + 2 O2 = CO2 + 2 H2O)
Why could the Bosch process be more favourable than the Sabatier process in long-term mission? What does its redox reaction look like?
→ Venting of CH4 using the Sabatier process involves the loss of H2, thus production of solid C via the Bosch reaction would be favourable
Reaction: CO2 + 2 H2 ⟺C(s) + 2 H2O
Electrolysis: H2O ⟺ H2 + ½ O2
Atmospheric CO2 (96% on Mars) is converted to methane and water at 300–400 °C and ~30 bar
What are the shortcomings of the single-stage Bosch process ?
slow reaction rates
fouling of catalyst by surface carbon
less favourable thermodynamics than Sabatier