L4: Oxidative Phosphorylation, ETCand ATP Synthase
1/30
Earn XP
Description and Tags
Flashcards covering oxidative phosphorylation, electron transport chains, reactive oxygen species, and ATP synthase mechanism based on the lecture notes.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What is the primary function of electron transport chains?
To transfer electrons from a donor to a terminal acceptor using a series of membrane-embedded protein and lipid carriers.
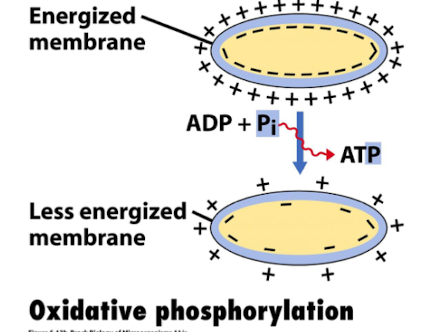
What is oxidative phosphorylation?
It is an oxygen-dependent process where cofactors NADH and FADH₂ are oxidised, and the energy generated from PMF is used to synthesise ATP via the ETC
What are the 4 main e- carriers types in the ETC?
Flavoproteins
Iron-sulfur proteins
Quinones
Cytochromes
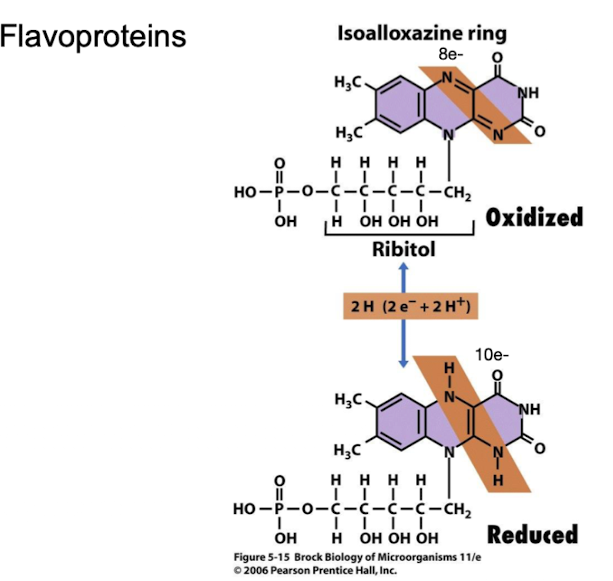
Which electron carriers are considered 'hydrogen carriers' and why?
Flavins and quinones, because they accept both protons and electrons.
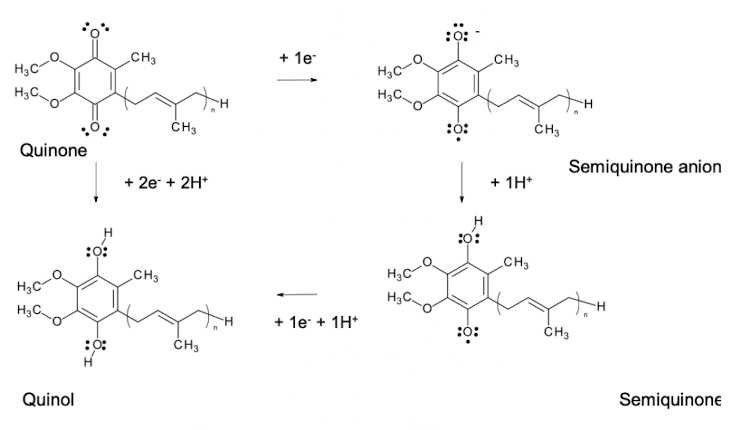
Which electron carriers accept electrons only?
Iron-sulphur proteins and cytochromes.
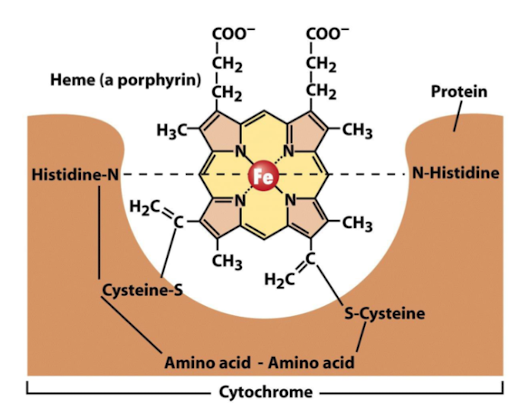
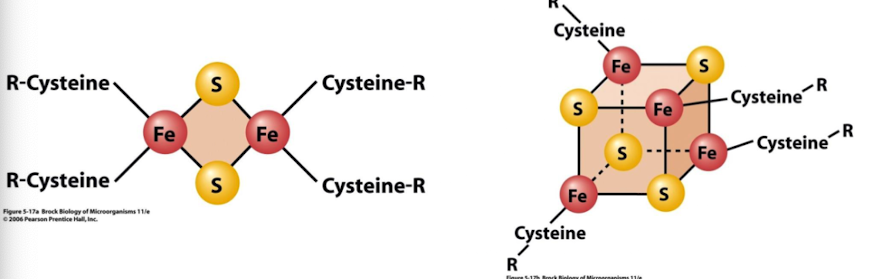
The redox potential of an FeS protein depends on _________
the type of cluster (2Fe2S or 4Fe4S) and the microenvironment within the protein.
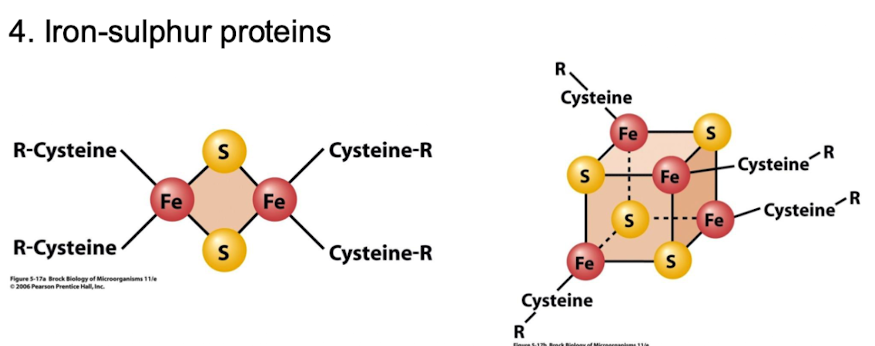
How many protein complexes are involved in oxidative phosphorylation?
Five:
Complex I (NADH dehydrogenase)
II (Succinate dehydrogenase)
III (Cytochromes bc1 + Fe-S proteins)
IV (Cytochrome c oxidase)
V (ATP synthase).
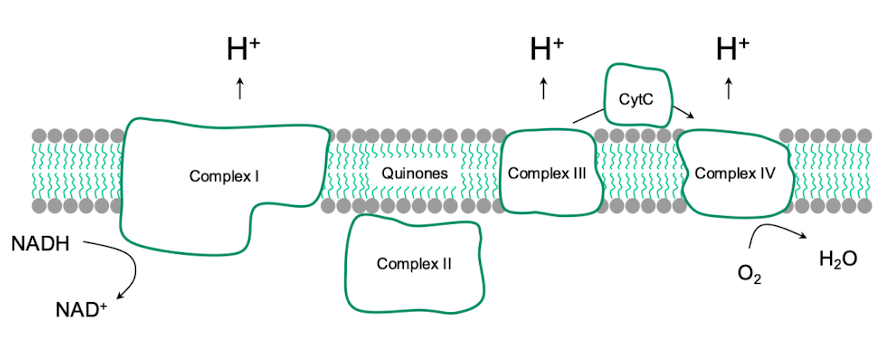
In bacterial electron transport chains, what is the role of Complex I?
It is NADH dehydrogenase, through which NADH-derived electrons enter the chain.
Contains FMN flavoprotein and Fe-S proteins
NADH is oxidized, e- are transferred to ubiquinone, and 4 H+ are pumped into the intermembrane space.
What is the function of Complex II in the bacterial electron transport chain?
(Succinate dehydrogenase)
It’s part of both the TCA cycle (oxidizing succinate to fumarate) and the ETC (transferring electrons via FADH2 to ubiquinone).
Doesn’t pump e- unlike Complex I

NADH-derived electrons enter the chain through ______ whereas succinate-derived electrons enter through ______.
complex I, complex II
*no direct electron transfer from complex I to complex II as they feed the quinone pool separately
What is complex III made up of?
A dimer of cytochrome b, cytochrome c1, and an iron-sulfur protein.
What is Complex III responsible for?
Reduced quinones e.g. QH2 channel electrons into complex III
It transfers electrons to cytochrome c while also pumping H+ into the intermembrane space, due to the supercharging from e- passed from the quinone pool
What is the significance of the Q cycle in the electron transport chain?
It increases the magnitude of the proton gradient by expelling 4 H+ and consuming 2 cytoplasmic H+ for every 4 e- transported through Complex III.
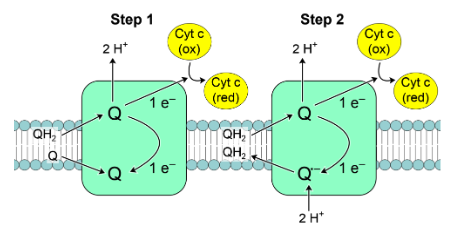
What are the different steps of the Q cycle?
When a reduced quinone QH2 feeds complex III, two H+ are expelled, one electron is transferred to a cytochrome, the other electron eventually passes to another quinone forming a quinone radical anion Q-
As the next QH2 feeds complex III, two H+ are expelled, one electron is transferred to a cytochrome
The other e- is taken up by Q- which takes up two cytoplasmic H+ to form another QH2, which can be re-used.
In this way, transport of four electrons results in export of four protons plus consumption of two further protons from the cytoplasm.
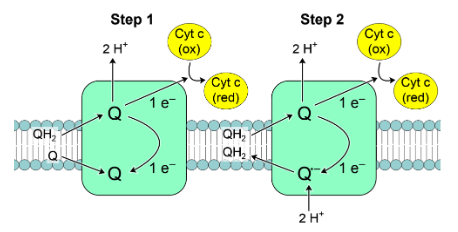
What occurs at Complex IV?
Terminal oxidase of ETC
It transfers electrons from Cytochrome c & reduces O2 → H2O
Has 2 Cu ion centres (Cua Cub) & 2 cytochromes
Electrons are passed through these 2 cyt with the nearby Cu ion transferring e- to O2 , reducing it to H2O
pumps 2 protons out of the matrix into the intermembrane space.
Which complexes contribute to proton pumping in the electron transport chain?
Complexes I, III, and IV
How does the flow of electrons down the electron transport chain generate proton motive force?
As electrons flow, the free energy released is used to pump protons out of the cell, creating an electrochemical gradient.
In aerobic respiration, what is the terminal electron acceptor and what is it reduced to?
Molecular oxygen (O2) is the terminal electron acceptor, reduced to H2O.
Name three partially reduced oxygen species (ROS) that can escape during aerobic respiration.
Superoxide radical, hydrogen peroxide, and hydroxyl radical.
How do reactive oxygen species cause damage to cells?
They are free radicals that can initiate reactions damaging DNA, proteins, and lipid molecules, and propagate further destructive radicals.
What is the role of antioxidants like vitamin C or flavonoids?
They form stable radicals that do not participate in chain reactions, allowing them to quench or neutralise more destructive radicals.
Which two enzymes do aerobic bacteria use to dispose of reactive oxygen species?
Superoxide dismutase and catalase.
Why can obligate anaerobes not tolerate oxygen?
They lack the enzymes (superoxide dismutase and catalase) necessary to dispose of reactive oxygen species.
How is ATP synthesised in the ETC?
Via Complex V (FoF1-ATP synthase) using phosphorylation using the proton gradient generated from Complexes I, III, and IV to drive ATP production.
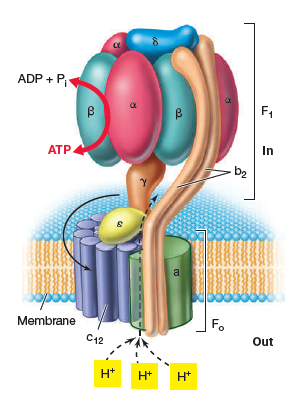
What are the two main components of ATP synthase?
The membrane-embedded Fo component: has 10-14 c subunits, 1 a and b subunit
The peripheral F1 component.
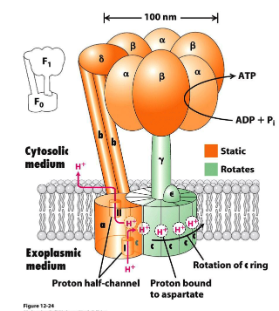
Which parts of the ATP synthase are static and which parts rotate?
Static: The 'a' subunit, 'b' subunit, and the α3β3 complex
Rotating: 'c' ring rotates and turns the 'γ' subunit.
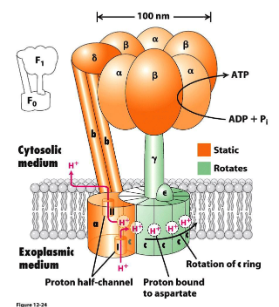
Describe the journey of a proton through the Fo component of ATP synthase to drive rotation.
A proton enters an outer half-channel of the 'a' subunit with 2 unconnected channels
The H+ neutralises an aspartate SC on a 'c' subunit, allowing the protonated 'c' subunit to rotate into the membrane's hydrophobic phase.
After a complete rotation, the protonated aspartate reaches an a subunit’s inner half-channel exposed to the cytosol
Proton is released into the cell & the deprotonated Asp can bind another proton to continue the cycle.
What 3 conformational changes occur in the β subunits by the rotation of the asymmetric γ subunit during ATP synthesis?
Each β subunit cycles through three states:
Loose binding of ADP and Pi
Tight binding where ADP and Pi condense to form ATP
Release of ATP.
______ through the a protein drives unidirectional rotation of the ____ which turns the ______ and ultimately drives ATP synthesis
Proton influx, unidirectional rotation of the c ring, which turns the γ subunit
How can ATP synthase function in reverse?
It can hydrolyze ATP to drive proton efflux, switching the direction of continuous rotation to generate PMF rather than consuming it.
For what purposes do bacteria surviving by fermentation still need proton motive force?
For flagellar rotation and for nutrient uptake by proton symport mechanisms.