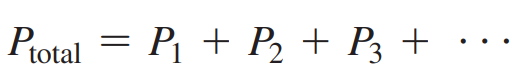Chapter 8: Gases
0.0(0)
0.0(0)
Card Sorting
1/20
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
1
New cards
Kinetic Molecular Theory of Gases
A model for the behavior of a gas that helps us understand gas behavior.
2
New cards
Pressure (P)
The force exerted by a gas against the walls of the container.
3
New cards
Volume (V)
The space occupied by a gas.
4
New cards
Temperature (T)
The determining factor of the kinetic energy and rate of motion of gas particles.
5
New cards
Amount (n)
The quantity of gas present in a container.
6
New cards
Boyle’s Law
The pressure increases if volume decreases; pressure decreases if volume increases.
7
New cards
Charles’s Law
As temperature of a gas increases, its volume increases; if its temperature decreases, volume decreases.
8
New cards
direct
A _____ relationship is one in which the related properties increase or decrease together.
9
New cards
Gay-Lussac’s Law
As temperature of a gas increases, its pressure increases; if its temperature decreases, pressure decreases.
10
New cards
The Combined Gas Law
All of the pressure–volume–temperature relationships for gases combined into a single relationship.
11
New cards
Avogadro’s Law
If the moles of gas are increased, the volume must increase; if the moles of gas are decreased, the volume must decrease.
12
New cards
0 °C (273 K)
Standard temperature
13
New cards
1 atm (760 mmHg)
Standard pressure
14
New cards
Partial Pressure
It is the pressure it would exert if it were the only gas in the container.
15
New cards
Dalton’s Law
States that the total pressure of a gas mixture is the sum of the partial pressures of the gases in the mixture.
16
New cards
Boyle’s Law Formula
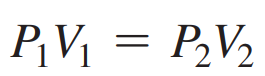
17
New cards
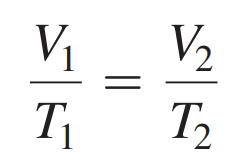
Charles’s Law Formula
18
New cards
Gay-Lussac’s Law Formula
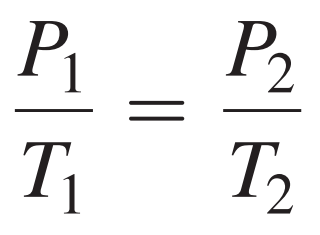
19
New cards
Combined Gas Law
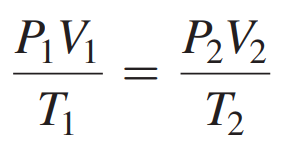
20
New cards
Avogadro’s Law Formula
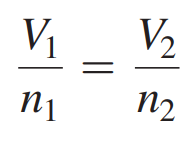
21
New cards
Partial Pressure Formula