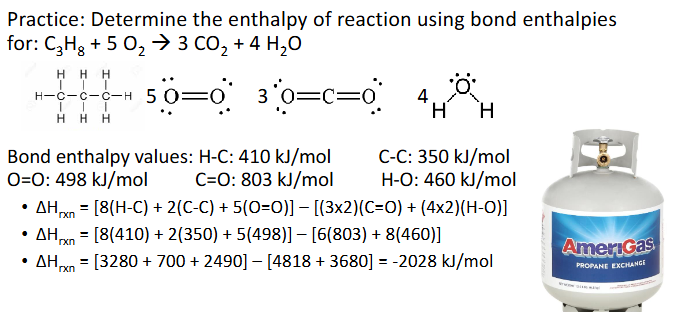Chapter 10 - Chemistry
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
Energy Types (Chapter 10)
Potential energy: stored energy in chemical bonds
Correlated to distance between particles and magnitude of charge or attraction
Kinetic energy: movement of particles
Correlated to temperature
Mechanical energy: total of potential and kinetic energy for an object
Energy Units (Chapter 10)
4 main energy units:
Joule, J: SI unit of energy
Kilojoule: 1000 J
calorie, cal: amount of energy required to raise the temperature of 1 g of water by 1°C, equal to 4.184 J
Calorie, Cal or kcal: unit of energy used in utrtition, 1 kcal = 1000 cal, 1 kcal = 4.184 kJ
Example:
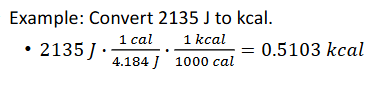
System & Surroundings (Chapter 10)
System: what is being studied, must be defined by experimenter
Surroundings: the environment around the system taht may exchange matter or energy with it
Types of systems:
Open: can exchange matter and energy with surroundings
Closed: can exchange energy with surroundings
Isolated: cannot exhcnage matter or energy with surroundings
System & Surroundings Examples (Chapter 10)
A pot with boiling water on the stove
Pot & water are system, stove and air around pot are surroundings, system is open if lid is off, closed if lid is on
A thermos with water and ice
Water, ice, & thermos are system, table and air around thermos are surroundings, system would be isolated if thermos could insulate perfectly (it can’t)
Work & Heat (Chapter 10)
Work, w: energy resulting from a force acting on an object over a distance
Heat, q: flow of energy that causes a chnage in temperature in an object or its surroundings
The sign of w & q are imporant and must be determined based on the situation
If work is done on the system (a force is exerted on it), w is positive
If a system is heated (energy flows to it), q is positive
If work is done by the system (a force is exterted by it), w is negative
If a system produces heat (energy flows form it), q is negative
Work & Heat Examples (Chapter 10)
Pot with boiling water on a stove
The system is heated, +q
A chair is pushed across the room
A force is exerted on the chair (system), +w

Work & Heat Practice (Chapter 10)
Answer

Law of Conservation of Energy (Chapter 10)
The first law of thermodynamics is the law of conservation of energy
Energy cannot be created or destroyed, but it can be transferred from one form to another
The energy of the universe is constant
Universe = system + surroundings
Internal Energy (Chapter 10)
Is the all the kinetic and potential energy of a system
Applies to movement of particles only
The chnage in internal energy is measured by the sum of heat and work
△U = q + w
If the internal energy of the system increases, △U is positive
If the internal energy of the system decreases, △U is negative

Internal Energy Practice (Chapter 10)
Answer

State & Path Functions (Chapter 10)
All quantitites we’ve discussed are function: heat, work, internal energy
State functions are independent of path; you can start in two different places and end up with the same answer; current state of system is described
U, internal energy
Path functions are depdent on path; if you start in different places, you will get different asnwers
q, heat
w, work
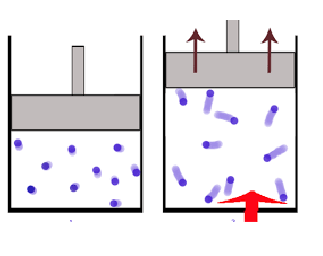
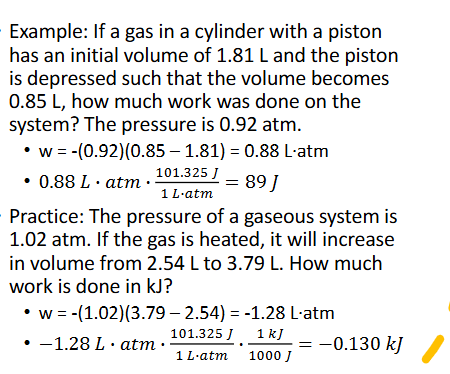
Pressure — Volume Work (Chapter 10)
Pressure — volume work is wokr done on or by a system where in the volume of a gas is increased or decreased agaisnt a constant pressure
w = -P △ V
△V = Vfinal - Vinitial
V is in L, P is in atm
1 L•atm = 101.325 J
Generally, systems are setup as gas in a clinder with piston that can move

Pressure — Volume Work Practice (Chapter 10)
Answer
Enthalpy & Heat Exchange (Chapter 10)
Chemists generally do reactions in open systems where in the pressure is constant (atmospheric pressure)
Heat exchange between system and surroundings is of most interest
Enthalpy, H, is teh combination of internal energy and P-V work
Wokr cancels out of the equation, leaving just qp - heat flow at constant pressure
△H = qp
Enthalpy has the same sign concentions as heat, but different vocabulary
Endotehrmic: heat absorbed, +△H
Exothermic: heat released, -△H

Heat Exchange Practice (Chapter 10)
Answer

Specific Heat (Chapter 10)
The specific heat, c, of a substance is the heat required to raise the temperature by 1°C
Unit: J/g°C
Difficult to change the temperature of subsatnces with high specific heats
Example: H2O has a very high specific heat of 4.184 J/g°C
The “specific heat equation” is used to calculate how much energu is required to chneg the temperature of agiven amount of specific substance
q = mc△T
m is mass in g
c is specific heat in J/g°C
△T is final — initial temperature (Tf - Ti) in °C
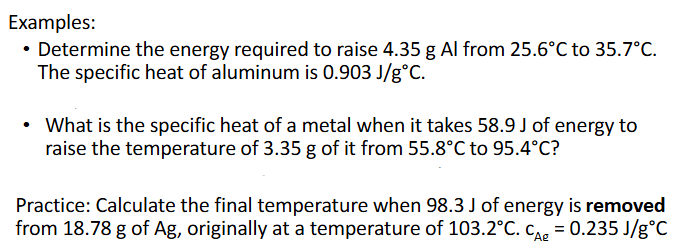
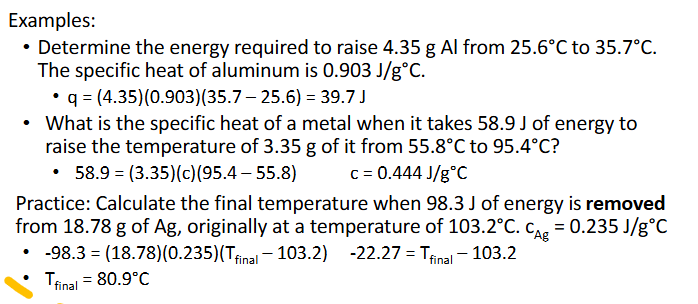
Specific Heat Practice (Chapter 10)
Answer
Calorimetry (Chapter 10)
In a calorimetry experiment, two susbatnces of different temperratures are put in contant with each other so the trnasfer of heat can be invetiagted
Metaal in water or mixxing solutions
Consatnt-prssure calorimetry - a chemical reaction atle spalce at sonatnt pressure or aolute is diddovled in as olvent
Constant-volume calorimetry - a chemical reaction takes place at constant volume in a a bomb calorimeter
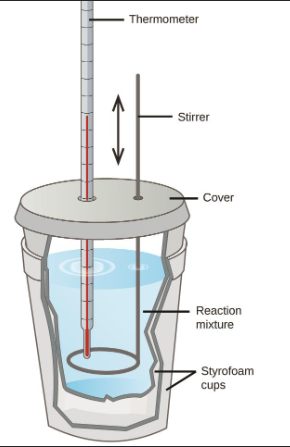
Metal in Water Calorimetry (Chapter 10)
General Process:
Metal is heated, typically in hot water
Room temperature water is added to calorimeter
Metal is added to colrimeter and temperature taken when thermal equilibrium is established
Example:

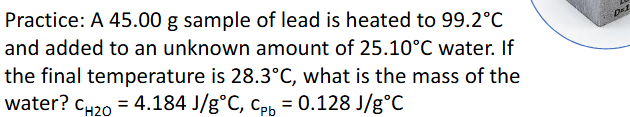
Metal in Water Calorimetry Practice Pt.1 (Chapter 10)
Answer
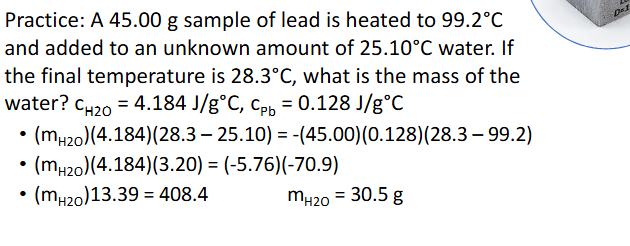

Metal in Water Calorimetry Practice Pt.2 (Chapter 10)
Answer
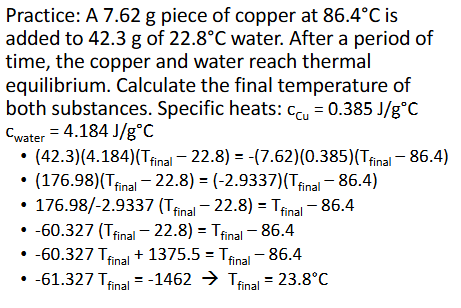
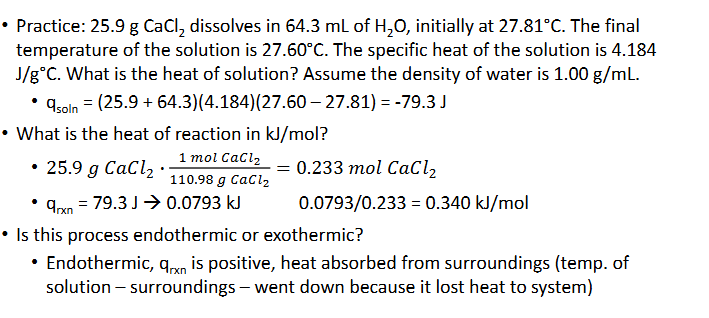
Constant-Pressure Calorimetry (Chapter 10)
The heat of solution, qsoln is what is measured in a calorimetry experiment wherein a substance dissolves
Most dissolving processes are either endothermic or exothermic, so a change in temperature can be measured as the solute dissolves in the solvent
The temperatures recorded will be of the solvent (initially) and teh solution (finally)
Since the dissolving process itself cannot have its temperature measured, it can be derived from that of the solution
qsoln = -qrxn (rxn = reaction)
Could also use qp for heat of reaction (P for constant-pressure)

Constant-Pressure Calorimetry Practice Pt.1 (Chapter 10)
Answer
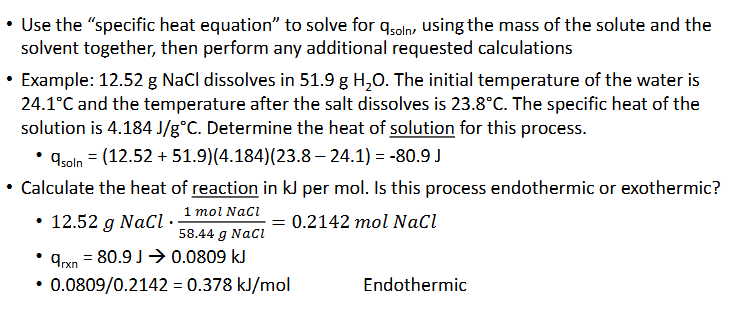

Constant-Pressure Calorimetry Practice Pt.2 (Chapter 10)
Answer
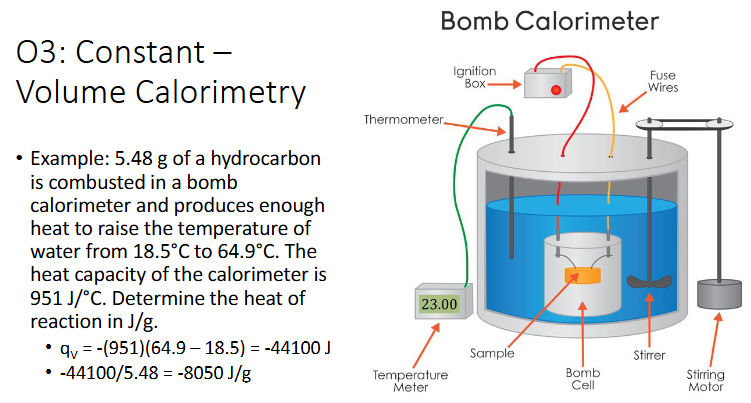
Constant-Volume Calorimetry (Chapter 10)
A bomb calorimeter is used to measure the heat of combustion of a reaction
Pressure is varibale and volume constant
The total heat capacity of the system includes the calorimeter itself, along with the water inside
You are given a value for this, Ccal, which applies to any process in that calorimeter with specific amoint of water
Calculate constant-volume heat of reaction using:
qv = -Ccal △T
qcomb can be used instead of qv
Example:
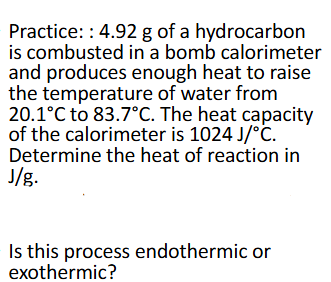
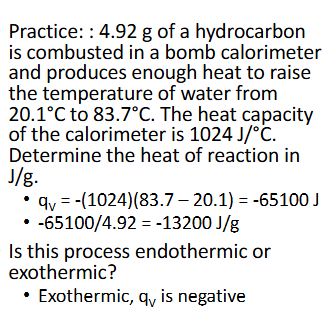
Constant-Volume Calorimetry Practice (Chapter 10)
Answer
Enthalpy as a Conversion Factor (Chapter 10)
When given the value for the entahlpy (heat released or gained) for a chemical reaction, it cna be related to the moles of each substance in that reaction
Example: formation of liquid water:
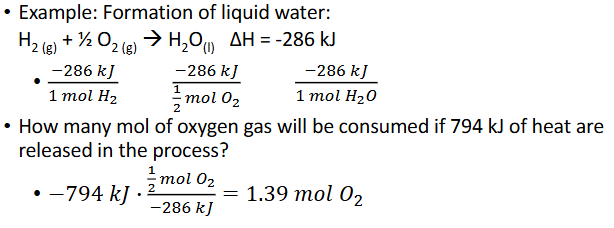

Enthalpy as a Conversion Factor Practice (Chapter 10)
Answer
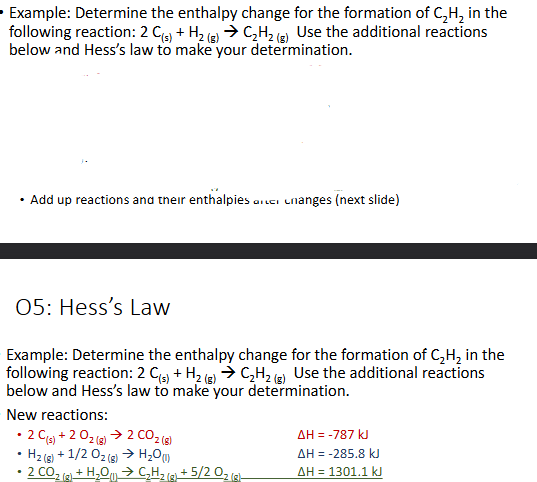
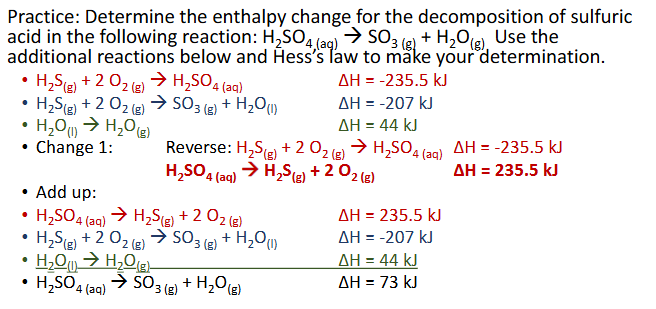
Hess’s Law (Chapter 10)
Hess’s law states that the enthalpies of 2 or more chemical reaction that can add up to an overall reaction, can also be added up to get the overall enthalpy
Similar to a system of equation in math where you manipulate one equation to cancel out a variable in both
The goal is to get the equations listed as steps to add up to be teh overall reaction by having certain substances cancel out. Cancellation occurs by one reaction having a subatnce as a reactant and another having it as a product
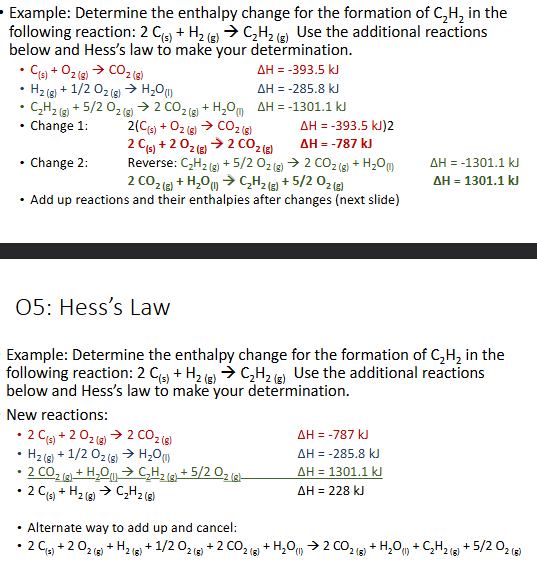
Hess’s Law Rules (Chapter 10)
Rules for chemical euqation manipulation:
You can;t chnage the chemicals in any way, just the coefficients
You can multiply or divide the coefficients as needed, but all coefficients in the equation must beaffected as well as the enthaply change
You can reverse a reaction by making the products the reactants and vice versam but the enthalpy chnage sign needs to be reversed as well
Substances can aptially cancel out if there are, for example, 2 mol A as a reactant and 1 mol A as a product in two of the reactions. This would result in 1 mol A as a reactant and no mol A as a product - like subtraction.
Hess’s Law Practice Pt. 1 (Chapter 10)
Answer
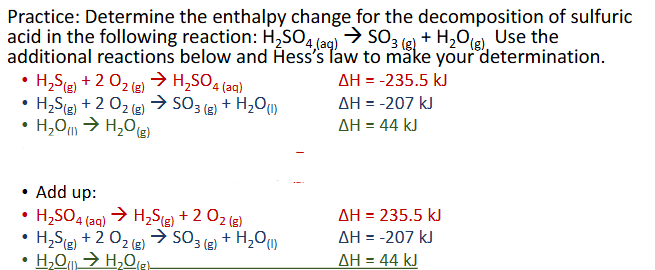
Hess’s Law Practice Pt. 2 (Chapter 10)
Answer
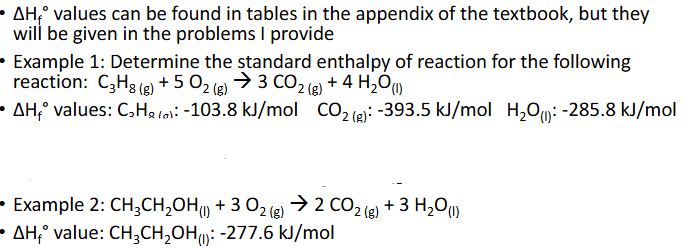
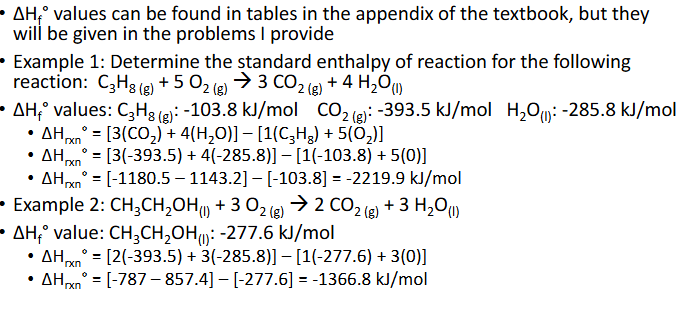
Enthalpy of Formation & Reaction (Chapter 10)
Enthalpy of formation, △Hf, is the amount of energy associated with forming 1 mol of a compounds from its elements
If there is a degree sign in the symbol (△Hf°), that means that this energy is associated with the standard state of the lement or teh state in which the susbatnce is most stabke at 25°C)
Can use enthalpies of formation to find the nehalpy of reaction, △Hrxn°
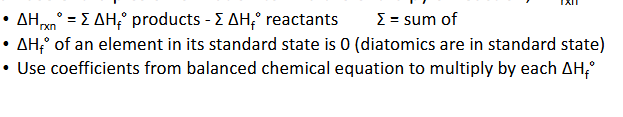
Enthalpy of Formation & Reaction Practice Pt. 1 (Chapter 10)
Answer
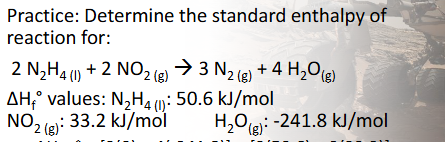
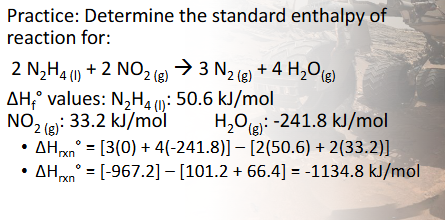
Enthalpy of Formation & Reaction Practice Pt. 2 (Chapter 10)
Answer
Bond Length & Strength (Chapter 10)
Bond length and strength are correlated
Shorter bonds are stronger, resulting in less reactive molecules
Longer bonds are weaker, resulting in more reactive molecules
Single bonds are longest, followed by double and the triple
Bond enthalpy is the enrgy associated with breaking a chemical bind in 1 mol of gas molecules
Bond breaking requires energy - endothermic
Bond making releases eegry - exothermic

Bond Enthalpies Example (Chapter 10)
Answer
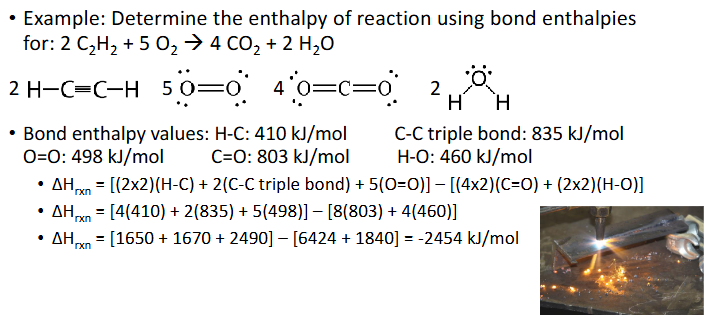
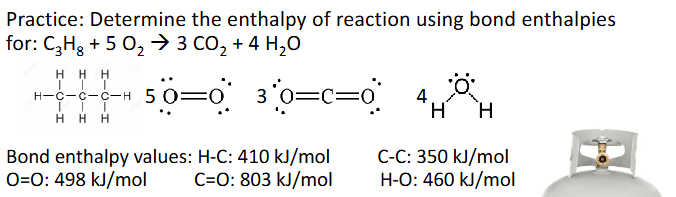
Bond Enthalpies Practice (Chapter 10)
Answer