Unit 3 The Atom >
3.1 Isotopic Notation
Ions and Isotopes
an ion is an atom or molecule with a charge, while an isotope is a form of an element with a different number of neutrons, therefore having a different mass number as well.
Atomic and Mass Numbers
An atomic number is the number of protons an atom has and determines the element’s place on the periodic table. The mass number is the number of neutrons and protons added
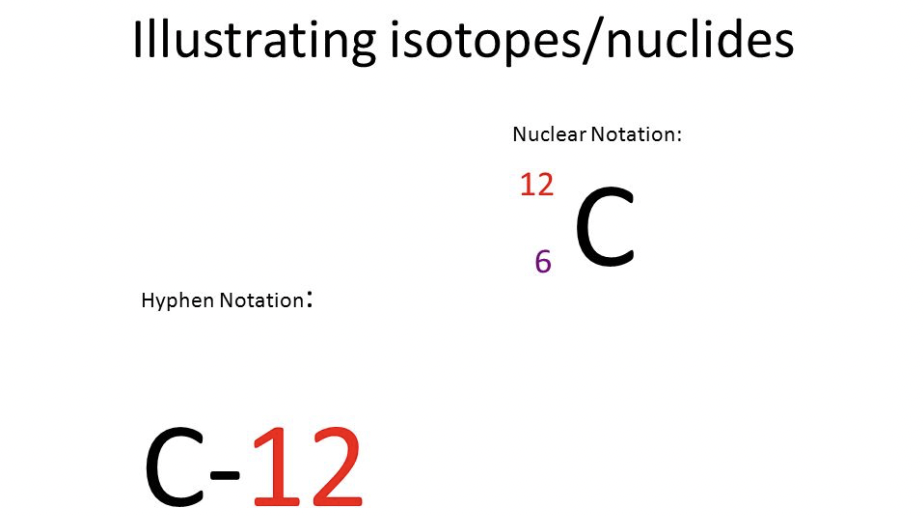
Nuclear and Hyphen Notation
Nuclear notation- Mass number over atomic number
Hyphen notation- Mass number
3.2 Relative Atomic Mass
Weighted Average
(Percent abundance of Isotope 1 x mass)+(Percent abundance of Isotope 2)…
note: when you get the percent abundance, convert the percentage into decimal form
Relative Atomic Mass
The mass number displayed on the periodic table
How to tell which element has the highest percent abundance= compare masses of all isotopes to the weighted average and see which isotope’s mass is the closest

3.3 Stability
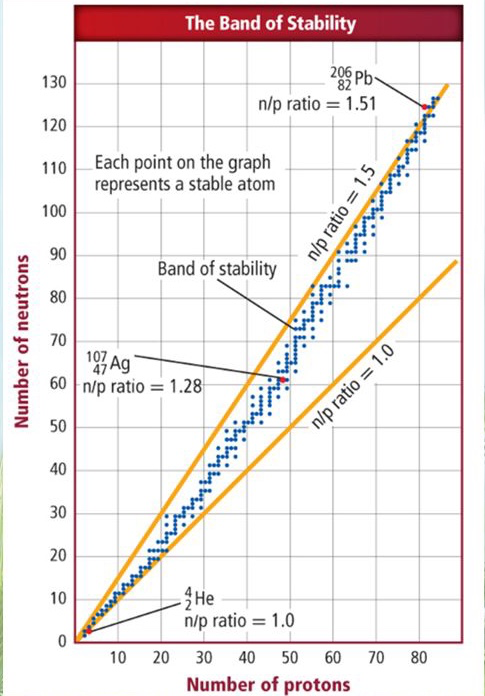
When an atom’s protons are above 82, then the element is unstable. Another factor deciding stability is: Elements with an atomic number of less than 20 can be determined stable if the nuclei have a 1 : 1 ratio (neutrons to protons). The ratio for elements with larger nuclei is 1.5 : 1 (neutrons to protons). Another way to tell if an element is stable is to locate it on the Band of Stability, if it is not one of the points marked, then it is not a stable isotope.
3.4 Natural and Artificial Radiation
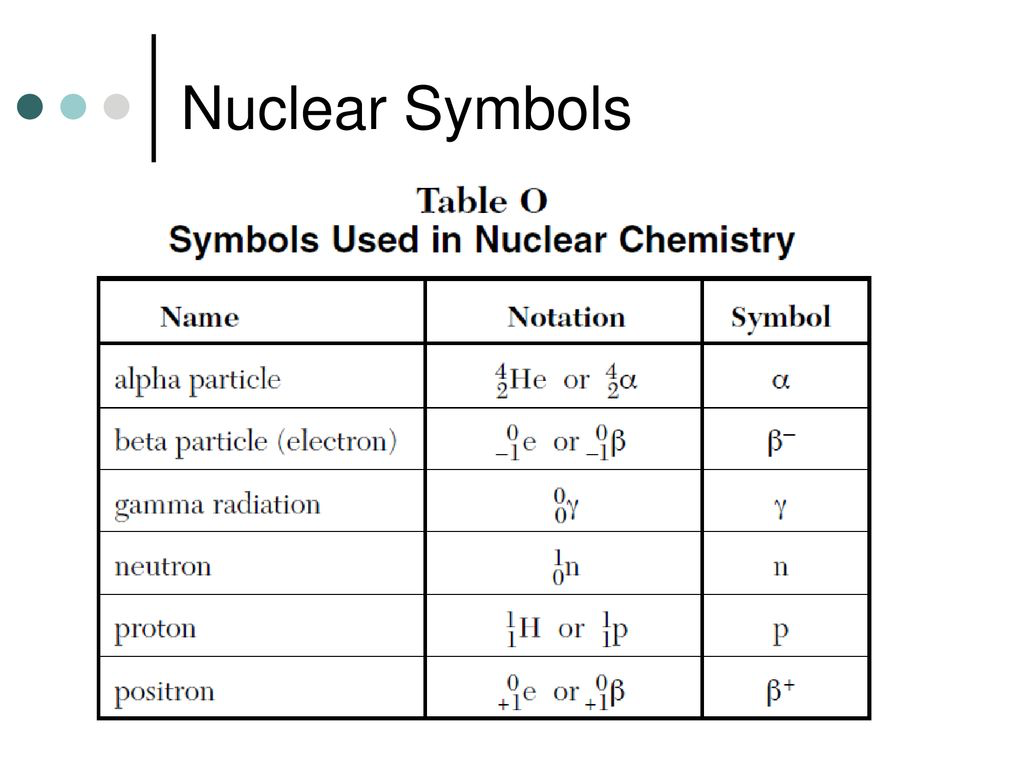
An atom's instability will cause it to decay and emit energy in the form of particles called alpha particles, beta particles, positrons, and gamma rays. Transmutation may occur from radioactive decay when a nucleus releases energy and causes the atom to transform into another isotope or element.
(The symbols and notation are listed below, as well as the types of material that the radioactive particles cannot pass through):
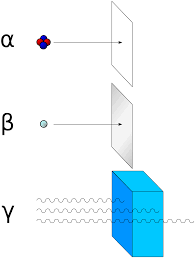
Nuclear equations:
A nuclear equation is organized with the target element on the left of the arrow, and on the right, a radioactive particle is added to another element.
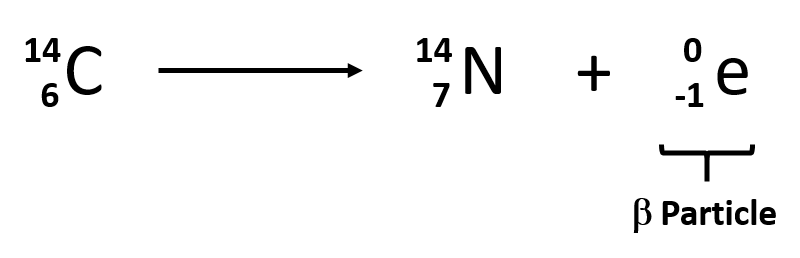
3.5 Half-Life
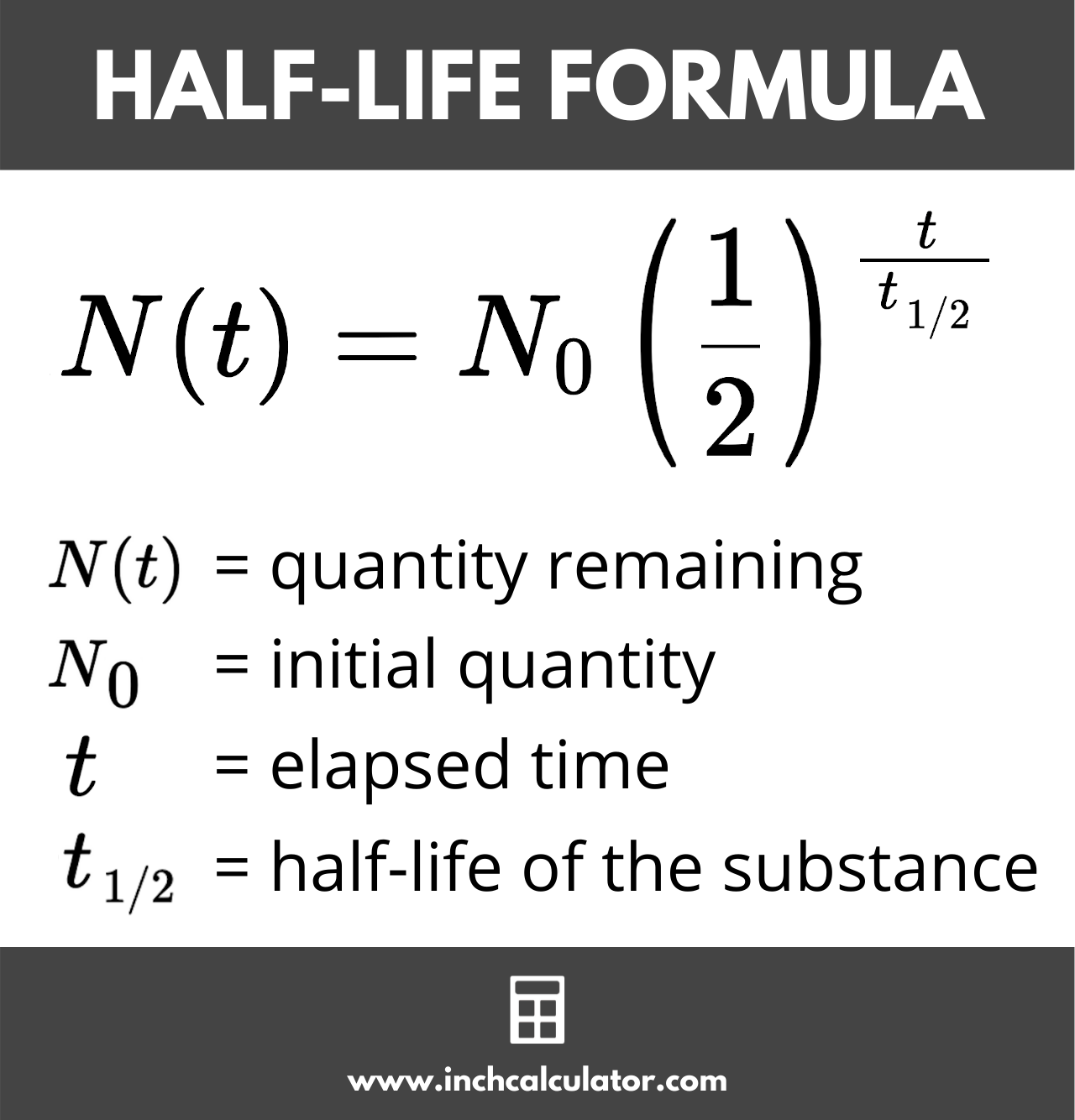
The Half-Life of an element is the time it takes for the radioactive sample to reduce to half its original quantity. It may take up to billions of years for this to happen, or as little as less than a second.
Remaining Mass= Initial mass x (1/2)^n
N= t/(half-life t)
N= cycles, t= time
Image representation:
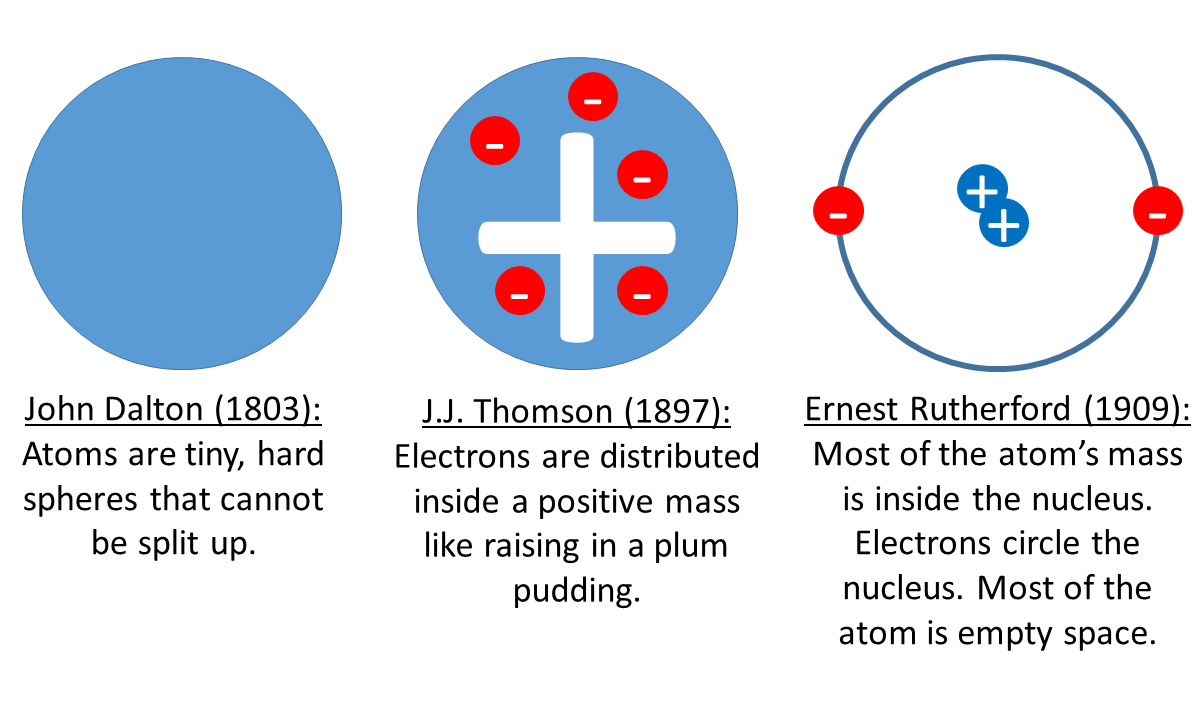
Scientists who contributed to the discovery of the atom