Reactions of acids and metal oxides
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Apparatus
Kettle
250cm3 beaker
100cm3 beaker
25cm3 measuring cylinder
Spatula
Glass rod
Heatproof mat
Universal indicator paper
Sulfuric acid
Copper(II) oxide on watch glass
Method
Add drop of sulfuric acid onto piece of universal indicator paper, strong acid
Use measuring cylinder, to place 25cm3 of sulfuric acid into small beaker
Fill large beaker about 1/3 full with hot water from kettle
Warm sulfuric acid beaker by letting it rest in hot water bath (2 minutes)
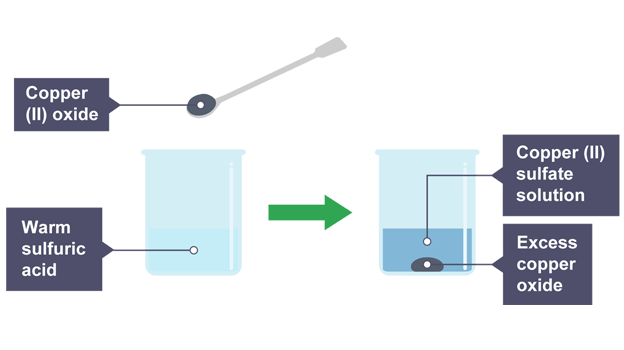
Carefully remove small beaker and add spatula of copper(II) oxide slowly and stir with glass rod
Keep adding copper(II) oxide, until some left at bottom of beaker
Let it sit for 2 minutes to allow black powder to settle
Use glass rod to add drop of solution from to universal indicator paper, now slightly acidic
Observations
At start of reaction pH of acid was 1-2
At end of reaction pH of solution was close to neutral
Blue solution produced (salt solution)
Heat produced (exothermic)
Most of black solid disappears
Equation
acid + metal oxide → salt + water
Error
adding too much acid
Safety
Sulfuric acid is corrosive
Wear safety goggles and gloves
Handle with care to avoid splashes