Physics - Particles
1/28
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
specific charge
Q/m
a particle’s charge to mass ratio
isotope
atoms of the same element with the same number of protons
use of carbon-14
it is radioactive to we use it to find the age of organic matter through carbon dating - calculate the % of C-14 remaining in an object and use the half life to therefore calculate age
strong nuclear force (SNF)
keeps nuclei stable by counteracting electrostatic repulsion
attraction and repulsion of SNF
repulsive up to 0.5fm
attractive from 0.5fm to 3fm
after 10fm, force becomes zero
unstable nuclei
have either too many protons or neutrons so SNF can’t keep them stable, therefore they decay to become more stable
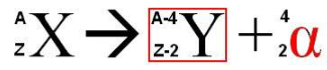
alpha decay
large nuclei with too many protons and neutrons
beta-minus decay
occurs in neutron-rich nuclei
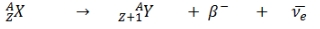
discovery of the antineutrino
at first scientists through beta minus decay only emitted an electron but observations of the energy of the beta particle emitted showed variation therefore another particle had to be emitted. Beta particles had a range of kinetic energies so another particle was emitted to conserve energy
antiparticles
every particle has an antiparticle of the same rest energy and mass but all other properties are opposite
pair production
a photon interacts with a nucleus/atom, creating a photon and it’s antiparticle
the minimum energy of the photon, hfmin = 2Eo (rest energy)
annihilation
when matter and antimatter meet, their mass is converted into 2 photons
the minimum energy of each photon, hfmin = Eo (rest energy)
uses of annihilation
PET scanners use positron emitting isotopes inside the patient that annihilate existing electrons, emitting gamma photons that can be easily detected
electromagnetic radiation
travels in packets called photons
E = hf = hc/λ
EM radiation emittance
emitted when electrons move shells, slow down, stop or change direction
laser beams
photons of the same frequency
power of beam = no. photons passing a point per second x f
four fundamental forces
gravity, electromagnetic, weak nuclear and strong nuclear
cause of fundamental force
exchange particles / bosons
strong nuclear interaction
exchange particle: gluon (between quarks) or pion (between nucleons)
relative strength: 1
range: 3×10-15m
acts on: hadrons
weak nuclear interaction
exchange particle: W boson - W+, W- , Wo
relative strength: 10-5
range: 10-18 m
rest mass: 81, 81, 93
acts on: all particles
electromagnetic interaction
exchange particle: virtual photon
relative strength: 10-2
range: infinite
acts on: charged particles
gravitational interaction
exchange particle: graviton
relative strength: 10-38
range: infinite
acts on: particles with mass
electron capture
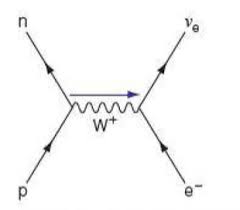
electron-proton collision
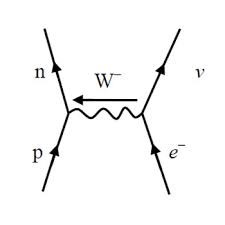
neutron-neutrino collision
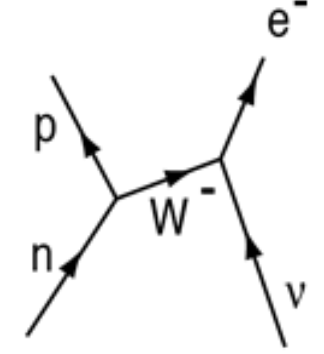
proton-antineutrino collision
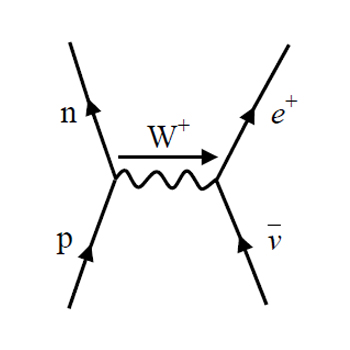
beta plus decay
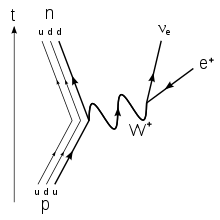
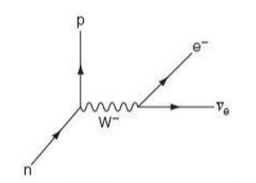
beta minus decay
leptons
e.g. electrons, muon neutrino
fundamental so don’t experience strong interaction as have no quarks