AP Chem Test 1A
1/12
Earn XP
Description and Tags
Topics 1.1 to 1.4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Methane, CH4, is the gas commonly found in labs to fuel Bunsen burners.
a) How many moles of methane are there in a 7.21 gram sample?
b) How many particles of methane are here in the sample?
c) How many atoms of hydrogen are found in the sample?
a) 0.450 mol
b) 2.71×10²³ p
c) 1.08×10²⁴ atoms
Helium, He, is used in balloons, deep sea diving tanks, and in industry. While it is the second most abundant element in the universe, in 2019 there was a shortage of helium which caused prices to rise. If 150. grams of helium is needed to cool a superconductor, how many atoms of helium are used?
2.24×10²⁵ atoms
What is the mass of 2.30×10²⁴ particles of water, H2O?
68.8 g
Which is a greater mass, 0.25 moles of carbon dioxide, CO2, or 1.5×10²³ particles of carbon monoxide, CO?
11 g CO2 > 7.0 g CO
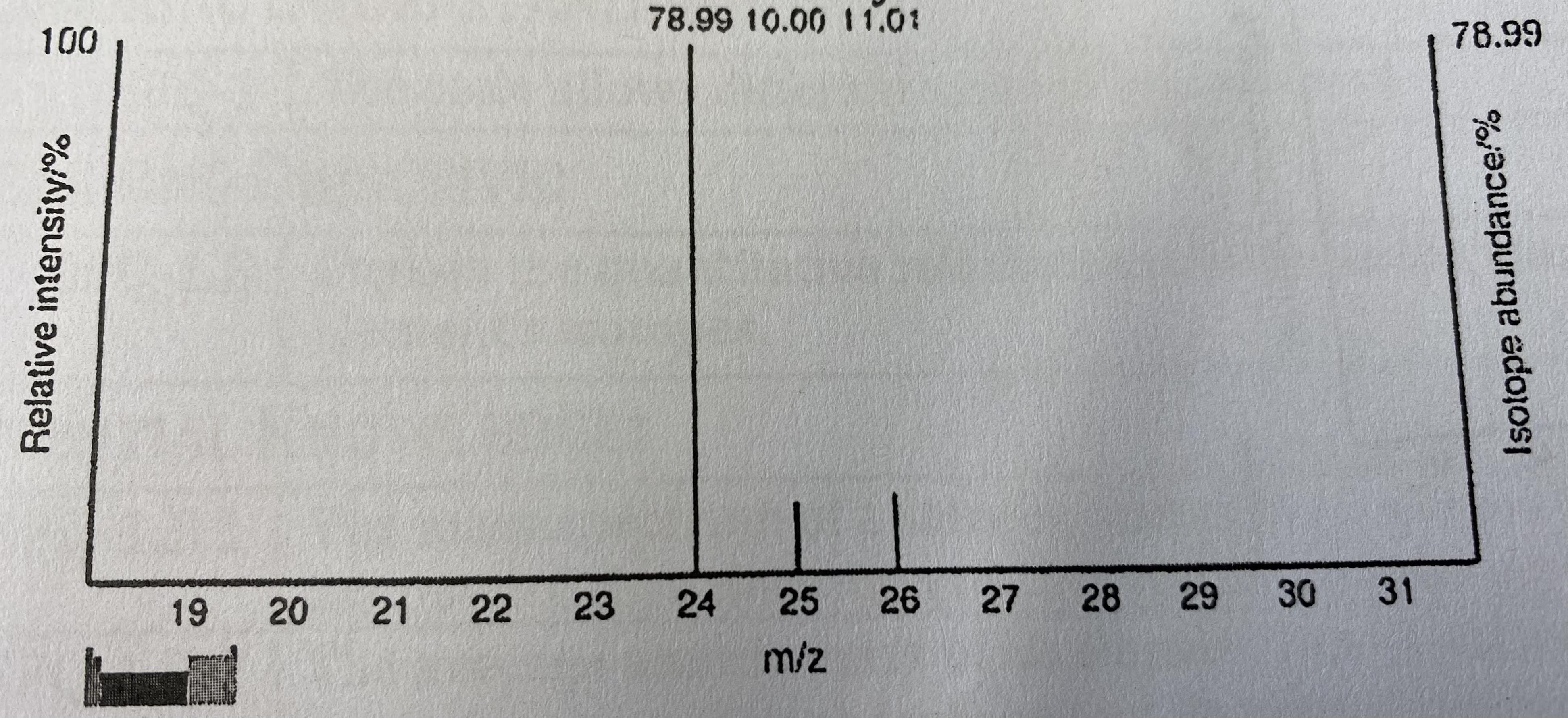
The mass spectrum of a sample of a pure element is given below. Calculate the average atomic mass of the element. What is the identity of the element?
24.3202 amu; magnesium (Mg)
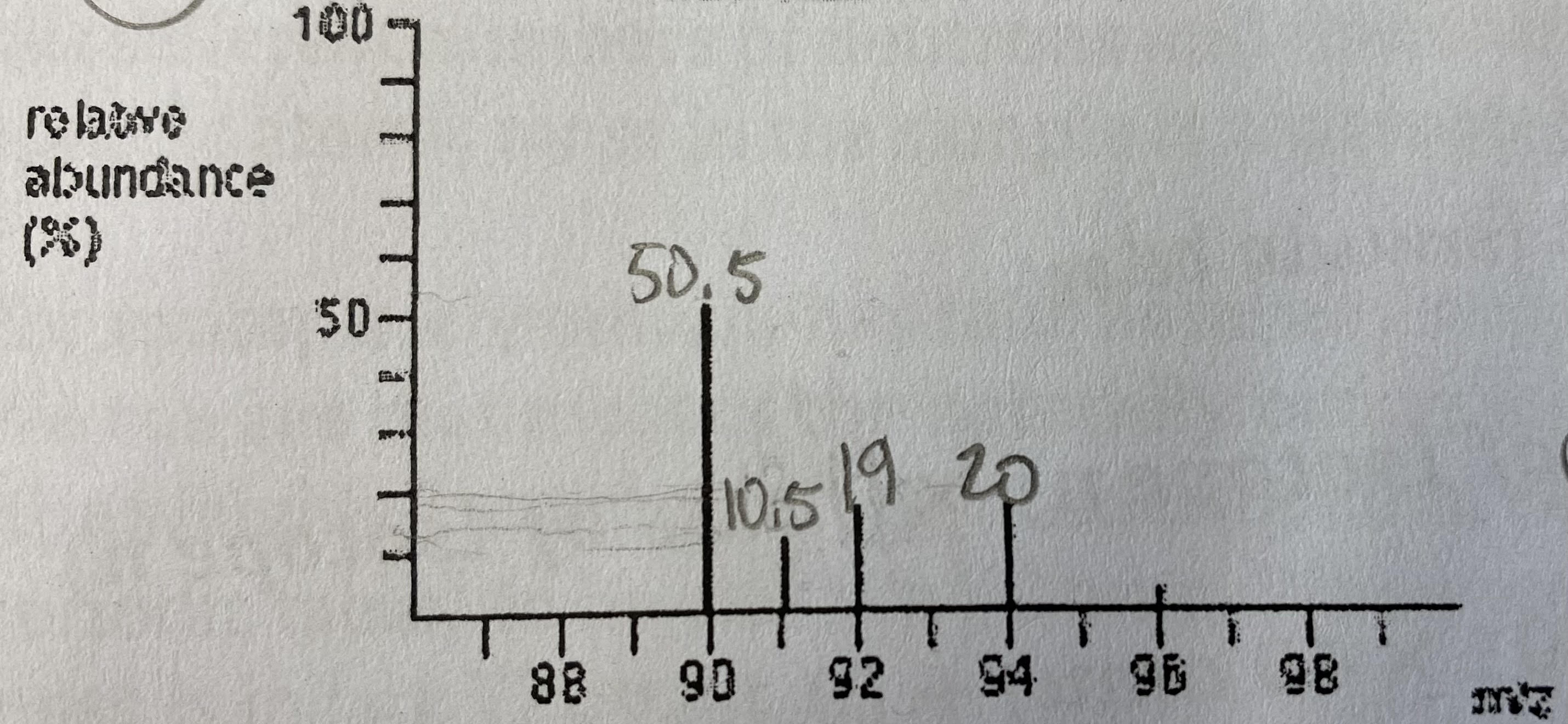
Determine the most likely element for the mass spectrum given below. Justify your choice.
91.285 amu; zirconium (Zr)
In the chemical closet, you found an unlabeled vial with a solid piece of an unknown element inside (element Z). You decided to put it in the mass-spec to figure out its atomic mass. The results showed that it has two naturally occurring isotopes, Z-85 and Z-87. Z-85 has a natural abundance of 72.17% and a mass of 84.912 amu. Z-87 has a natural abundance of 27.83% and a mass of 86.909 amu. Calculate the average atomic mass and determine the identity of mystery element Z.
85.468 amu, rubidium (Rb)
The most abundant molecule found in the human body is 88.810% oxygen and 11.190% hydrogen. Calculate the empirical formula for this substance.
H2O
Arginine is one of the amino acids; it is used in the biosynthesis of proteins. Analysis revealed that a sample of arginine was 41.368% carbon, 8.101% hydrogen, 32.162% nitrogen and 18.369% oxygen.
a) What is the empirical formula of arginine?
b) The molecular weight of arginine is 174.204 grams/mole. What is the molecular formula?
a) C3H7N2O
b) C6H14N4O2
A compound containing phosphorus and oxygen is a powerful dessicant. The compound is 43.642% phosphorus and 56.358% oxygen.
a) Calculate the empirical formula for this compound.
b) The molar mass of this compound is 283.889044 g/mol, determine the molecular formula.
a) P2O5
b) P4O10
Iron can form three different oxides, FeO, Fe2O3, and Fe3O4. A sample of iron oxide was analyzed and was found to contain 69.943% iron with the rest of the mass from oxygen. Determine the empirical formula to determine the identity of the iron oxide.
Fe2O3
A 15.0 gram sample of sodium hydrogen carbonate, NaHCO3, was contaminated with an impurity. In order to determine the purity of the sample, it was heated to decompose the material according to the following reaction: 2NaHCO3—>Na2CO3 + H2O + CO2. If 6.35 grams of sodium carbonate, Na2CO3, were recovered, what percentage (by mass) of the sample was sodium hydrogen carbonate, NaHCO3?
61.7%
A sample of brass weighing 1.203 grams was analyzed. Brass is an alloy composed of copper, Cu, and zinc, Zn. The zinc in the alloy was reacted with 35.123 grams of hydrochloric acid, HCl, in excess, according to the following balanced equation: Zn + 2HCl —> H2 + ZnCl2. After all of the zinc reacted, the mass of the remaining solution wieghed 36.309 grams.
a) What mass of hydrogen gas was produced?
b) What mass of zinc reacted?
c) What was the percentage of zinc (by mass) in the alloy?
a) 0.017 g
b) 0.55 g
c) 46%