PMI 128 Lecture 8-10
1/136
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
Viral Pathogenesis
Mechanisms by which viruses cause diseases
Host-Virus Interaction Importance
Critical for understanding viral disease mechanisms
Factors that are involved in Viral Pathogenesis
Innate Immunity: 1st line of defense against viral infections
Inflammation: Body’s response to infection, causing tissue damage
Adaptive immunity: Specific immune response developed after exposure
Viral Replication/tropism: The ability of a virus to infect specific cells and reproduce within the host.
Philosophy of Parasitism
To persist in a population, a parasite cannot kill its current host before its progeny has been transmitted to the next host
Four steps for animal viruses to be successful parasites
Entry into host, Dissemination within the host, Shedding from the host, transmission to the next host
Differences in viruses and their steps for being successful parasites
Not all viruses go thorough all the phases of entry→dissemmination→ shedding-transmission
Some can be successful by entry/entry and dissemination/etc…
Viral dissemination
is the process by which viruses spread throughout the host's body after initial entry.
Once it enters, it can replicate locally and then disseminate (spread) to other tissues or organs
Viral dissemination and compartmentalization
Dissemination allows the virus to reach new areas and Compartmentalization is after, which is when the virus becomes isolated and maybe genetically distinct in those sites
Different routes of viral entry into the host
Respiratory tract
Alimentary tract
Urogenital tract
Eyes
Skin
The 2 main ways of Entry into the Host
Mucus Membrane and Transcutaneous and Intravenous Injection
Mucus Membrane pathways
Respiratory tract
Gastrointestinal tract
Urogenital tract
Respiratory tract examples (Entry into Host)
Influenza
Rhinoviruses
Gastrointestinal tract examples (Entry into Host)
Poliomyelitis
Rotavirus
Urogenital tract examples (Entry into Host)
HSV
HPV
HIV
Transcutaneous and Intravenous Injection pathways (Entry into host)
Arboviruses
Intramuscular
Intravenous/transfusion
Intramuscular examples (Entry of Host)
Rabies
Intravenous/transfusion examples (Entry of Host)
HIV
Hepatitis B
Routes of virus entry into the GI tract
M cells
M cells
Microfold cells
facilitate the transport of antigens from the gut lumen to the immune cells, playing a critical role in the immune response.
M cell transport of virions
M cells are specialized epithelial cells in the GI tract, mainly located over Peyer’s patches.
They transport particles (including viruses) from the intestinal lumen to immune cells beneath the epithelium.
Virions exploit M cells by being taken up and delivered across the epithelial barrier, gaining access to underlying tissues and lymphoid structures.
This allows viruses to bypass normal defenses and spread to deeper parts of the body.
Shortening of Villi in Viral Infections
Some GI viruses (e.g., rotavirus, norovirus) cause damage to intestinal villi—the small finger-like projections that absorb nutrients.
This leads to shortening or blunting, reducing the surface area for absorption.
Result: Malabsorption, diarrhea, and nutrient loss.
Often accompanied by death of epithelial cells and inflammation.
Rotavirus infection of small intestinal enterocytes
Rotavirus infects mature enterocytes lining the tips of the villi in the small intestine.
It causes cell death, leading to villus blunting, reduced absorption, and leakage of fluid into the gut lumen.
This results in watery diarrhea, electrolyte imbalance, and dehydration.
Cleavage of Influenza HAO by Tryptase Clara
The process by which Tryptase Clara, an enzyme found in the respiratory tract, cleaves the hemagglutinin (HA) protein of the Influenza virus. This cleavage is essential for viral activation and infectivity, enabling the virus to effectively enter host cells.
This enzyme facilitates the activation of the virus, allowing it to infect respiratory epithelial cells effectively.
What triggers the immune response in Sars-COV-2 infection?
infection of epithelial cells by the virus leads to virus replication and release, triggering macrophage activation
How are macrophages involved in the Sars-COV-2 cytokine storm?
Macrophages detect viral peptides and become activated, presenting antigens to T cells and releasing pro-inflammatory cytokines
Which can contribute to widespread inflammation and immune overactivation, resulting in tissue damage and severe respiratory symptoms.
What role do T-cells play in the cytokine storm of COVID-19?
Activated T cells release additional proinflammatory cytokines, amplifying the immune response
What causes the “cytokine storm” in COVID-19?
An uncontrolled, exuberant immune response from T cells and macrophages leads to excessive cytokine and chemoattractant release.
This hyper-inflammatory response results in widespread tissue damage and severe complications associated with COVID-19.
What are the effects of proinflammatory cytokines during SARS-CoV-2 infection?
They attract immune cells, cause tissue damage, and contribute to acute respiratory distress syndrome (ARDS).
What is Acute Respiratory Distress Syndrome (ARDS) in COVID-19?
A severe condition caused by cytokine storm, leading to:
Necrosis
Tissue destruction
Leukocyte influx
Blood vessel dilation
How does Varicella-Zoster Virus (VZV) enter the body?
Enters through the conjunctiva or upper respiratory tract.
Where does VZV first replicate after entering the body?
In the primary lymph nodes, around day 0 to day 4–6, leading to primary viremia.
What is primary viremia in VZV infection?
It is the initial spread of the virus through the bloodstream after replication in lymph nodes.
Where does VZV replicate during the secondary viremia stage?
In the liver, spleen, and other organs before spreading further.
When does the chickenpox rash typically appear?
Around day 14, after secondary viremia, when the virus infects the skin.
What happens to VZV after the acute infection resolves?
It becomes latent in sensory ganglia by infecting sensory neurons and satellite cells.
What is reactivation of VZV?
Reactivation of latent VZV leads to shingles (herpes zoster), typically later in life.
What is an enterocyte?
A type of epithelial cell lining the intestines, responsible for nutrient absorption and a primary target of rotavirus infection
What is NSP4?
A rotavirus-encoded nonstructural protein that disrupts calcium homeostasis, tight junctions, and activates the enteric nervous system, contributing to diarrhea.
What does [Ca²⁺]i refer to in cell biology?
The concentration of free calcium ions inside the cytoplasm of a cell.
What is the endoplasmic reticulum (ER)?
An organelle that serves as a major calcium storage site in cells and plays a role in protein and lipid synthesis.
What is a viroplasm (Vi)?
A specialized structure in virus-infected cells where viral replication and assembly occur.
What are tight junctions?
Protein complexes that seal neighboring epithelial cells together to prevent leakage of water and solutes between them.
What is the enteric nervous system (ENS)?
A network of neurons in the gut wall that controls gastrointestinal functions such as secretion and motility
What is paracellular transport?
The movement of substances across the epithelium through the space between cells, rather than through the cells.
What is phospholipase C (PLC)?
An enzyme that hydrolyzes membrane lipids to generate IP₃, which releases Ca²⁺ from intracellular stores.
What is inositol trisphosphate (IP₃)?
A signaling molecule that binds to receptors on the ER, triggering the release of stored calcium into the cytoplasm.
What are crypt cells in the intestine?
Cells located in the intestinal crypts responsible for fluid secretion and cell regeneration.
What is integrin α2β1?
A cell surface receptor that can bind NSP4, playing a role in rotavirus-induced diarrhea, especially in neonatal mice.
What viral protein is key to the mechanism by which rotaviruses cause diarrhea?
NSP4
What is the initial cellular outcome after rotavirus infection of enterocytes?
Virus entry, replication, and release of NSP4 and virus particles; NSP4 increases intracellular Ca²⁺ levels.
What is the role of viroplasms (Vi) in rotavirus-infected cells?
Sites of viral replication and assembly within the host cell.
Through what kind of secretory pathway is NSP4 released from infected cells?
A nonclassical secretory pathway.
How does NSP4 lead to increased intracellular calcium ([Ca²⁺]i) in infected cells?
By inducing calcium release from internal stores, primarily the endoplasmic reticulum.
How does NSP4 affect tight junctions in infected cells?
It disrupts tight junctions, allowing paracellular flow of water and electrolytes
What signaling cascade does extracellular NSP4 activate in uninfected cells?
It binds to a receptor, triggering PLC activation and IP₃ generation, leading to Ca²⁺ release.
What second messenger does PLC produce that contributes to calcium release?
Inositol trisphosphate (IP₃).
What cellular organelle primarily stores the Ca²⁺ that NSP4 helps release?
The endoplasmic reticulum (ER).
What structural change does increased intracellular calcium cause in enterocytes?
Disruption of the microvillar cytoskeleton.
How does increased [Ca²⁺]i lead to Cl⁻ secretion?
It activates ion channels that drive chloride out of the cell into the lumen, drawing water with it
How does NSP4 or elevated Ca²⁺ levels affect crypt cells?
They stimulate crypt cells directly or via ENS activation, leading to Cl⁻ and water secretion.
What is the sequence of cellular events starting from virus infection to diarrhea?
Infection → NSP4 release → Ca²⁺ release → cytoskeleton disruption + ENS stimulation → ion/water secretion → diarrhea.
What role does the ENS play in rotavirus-induced diarrhea?
It receives signals from infected villus cells and stimulates secretion by crypt cells.
What does the activation of the ENS by NSP4 signify about rotavirus pathogenesis?
It involves both direct cellular disruption and indirect neural signaling.
How do infected enterocytes communicate with the ENS?
By basolateral release of NSP4 and/or effector molecules that stimulate enteric neurons.
Can uninfected neighboring cells be affected by NSP4? How?
Yes; extracellular NSP4 binds to receptors, increasing [Ca²⁺]i and contributing to secretion and barrier dysfunction.
Why is α2β1 integrin significant in neonatal rotavirus infection?
It binds NSP4 and triggers diarrhea in neonatal mice, indicating a receptor-mediated effect.
What are the main types of viral spread during dissemination within the host?
Local Spread
Viremia
Neural Spread
What is viremia?
The presence of viruses in the bloodstream, allowing for systemic infection and spread to various organs.
What are the two types of viremia?
Cell-associated
Plasma
What is Plasma Viremia?
The virus is freely circulating in the blood plasma
Meaning: Rapid spread, but easier to kill
What is Cell-associated Viremia?
The virus is inside or attached to blood cells, such as white or red blood cells
Meaning: Protected transport, evades immunity
What are the sources of viremia?
Lymphatics
Endothelium
Blood cells
What is neural spread in viral pathogenesis?
Spread of the virus through the nervous system
What are two directions of neural viral spread?
Centripetal (retrograde)
Centrifugal (anterograde)
Which viruses are associated with centripetal (retrograde) neural spread?
Rabies
HSV (Herpes Simplex Virus)
What is Centripetal (retrograde) spread?
Movement of the virus toward the central nervous system (CNS)
Pathway: from peripheral nerves → spinal cord/brain
What is Centripetal (retrograde) spread?
Movement of the virus away from the central nervous system (CNS)
Pathway: from CNS or ganglia→ skin, mucosa, or other peripheral tissues
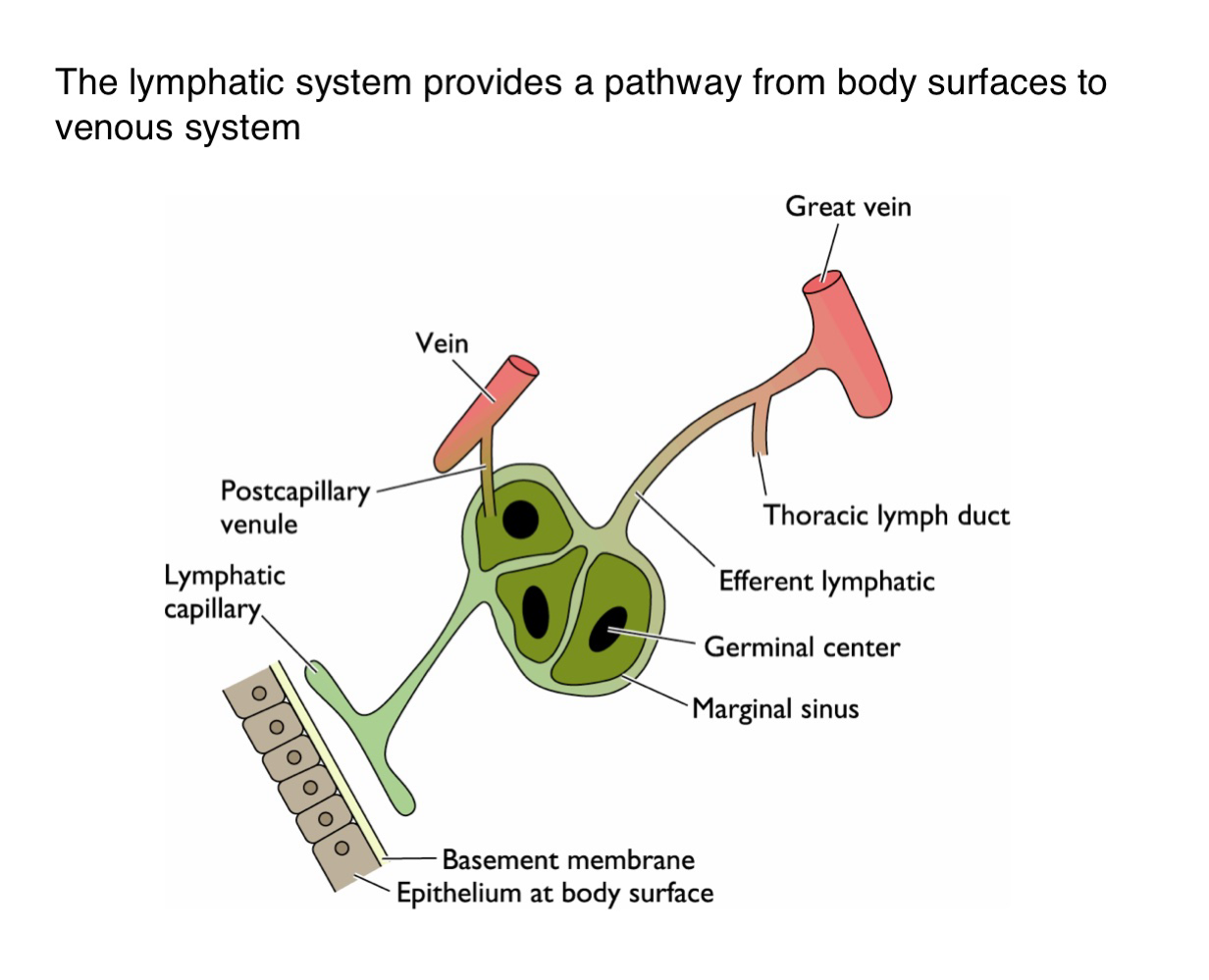
What is the function of the lymphatic system in viral dissemination?
It serves as a conduit for viruses to spread from epithelial surfaces to the venous circulation via lymphatic vessels
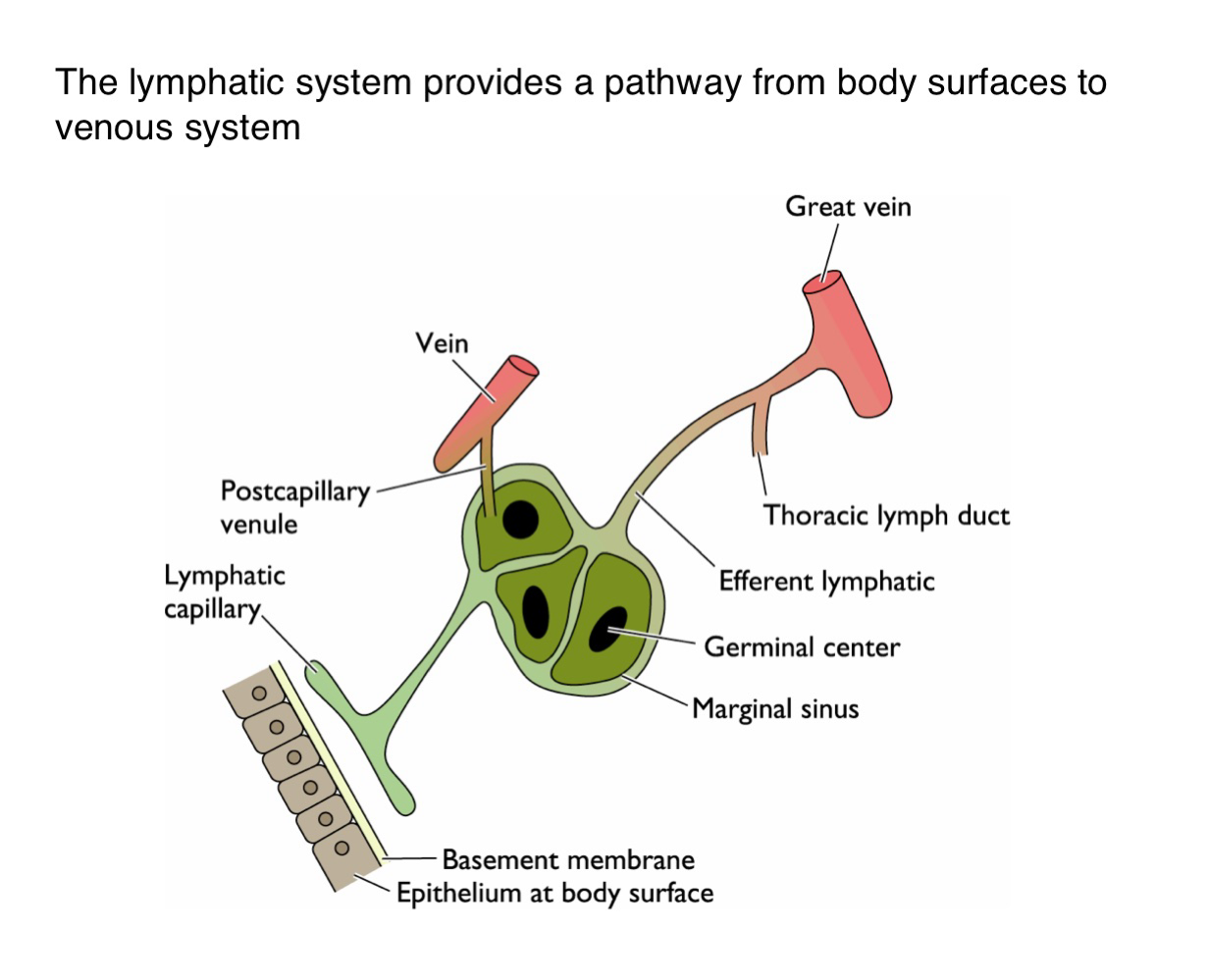
How do viruses gain access to the lymphatic system from the body surface?
Viruses penetrate the basement membrane beneath the epithelium and enter lymphatic capillaries
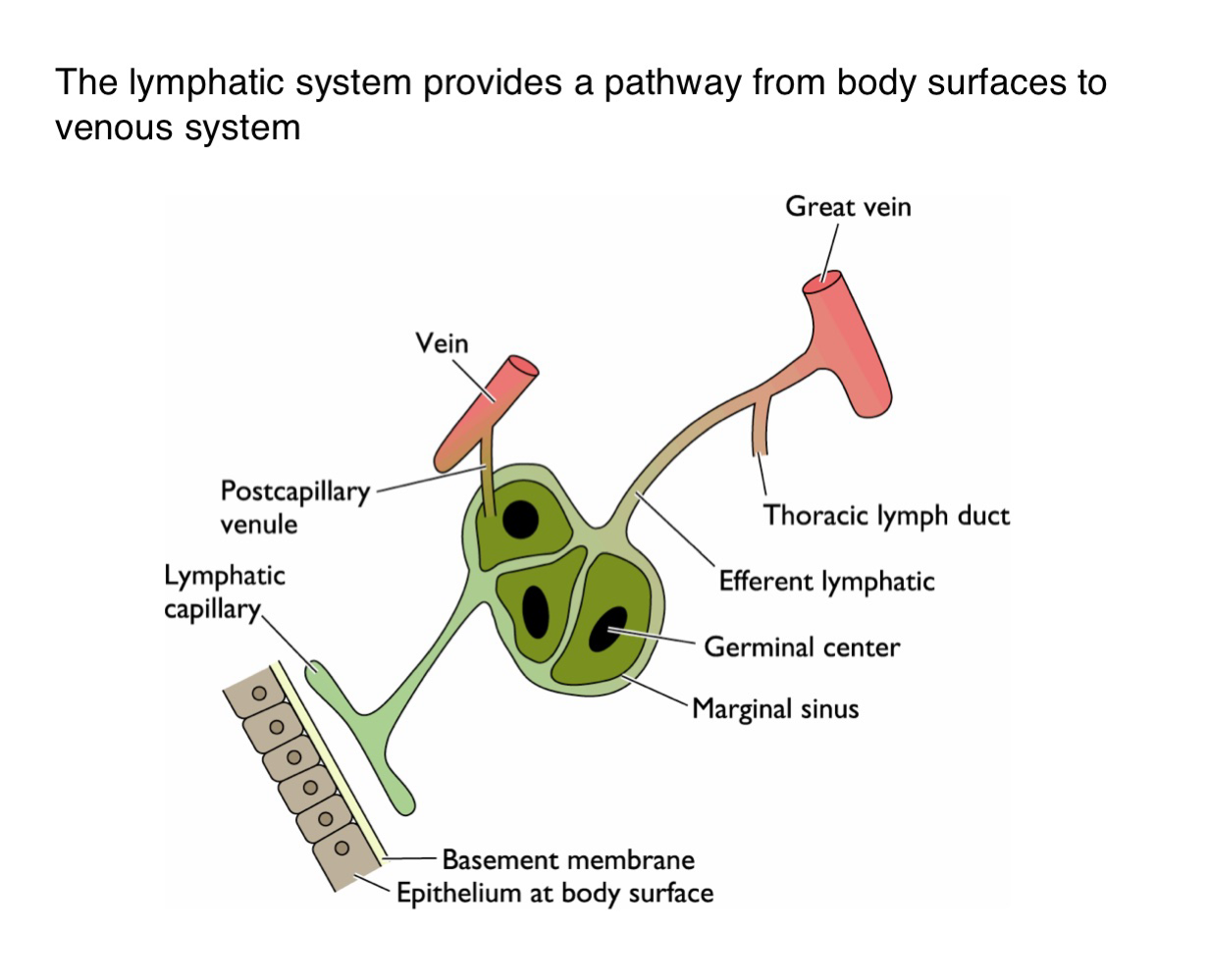
What happens to viruses one they enter the lymphatic capillaries?
They are transported to regional lymph nodes where they may replicate in immune cells such as those in germinal centers
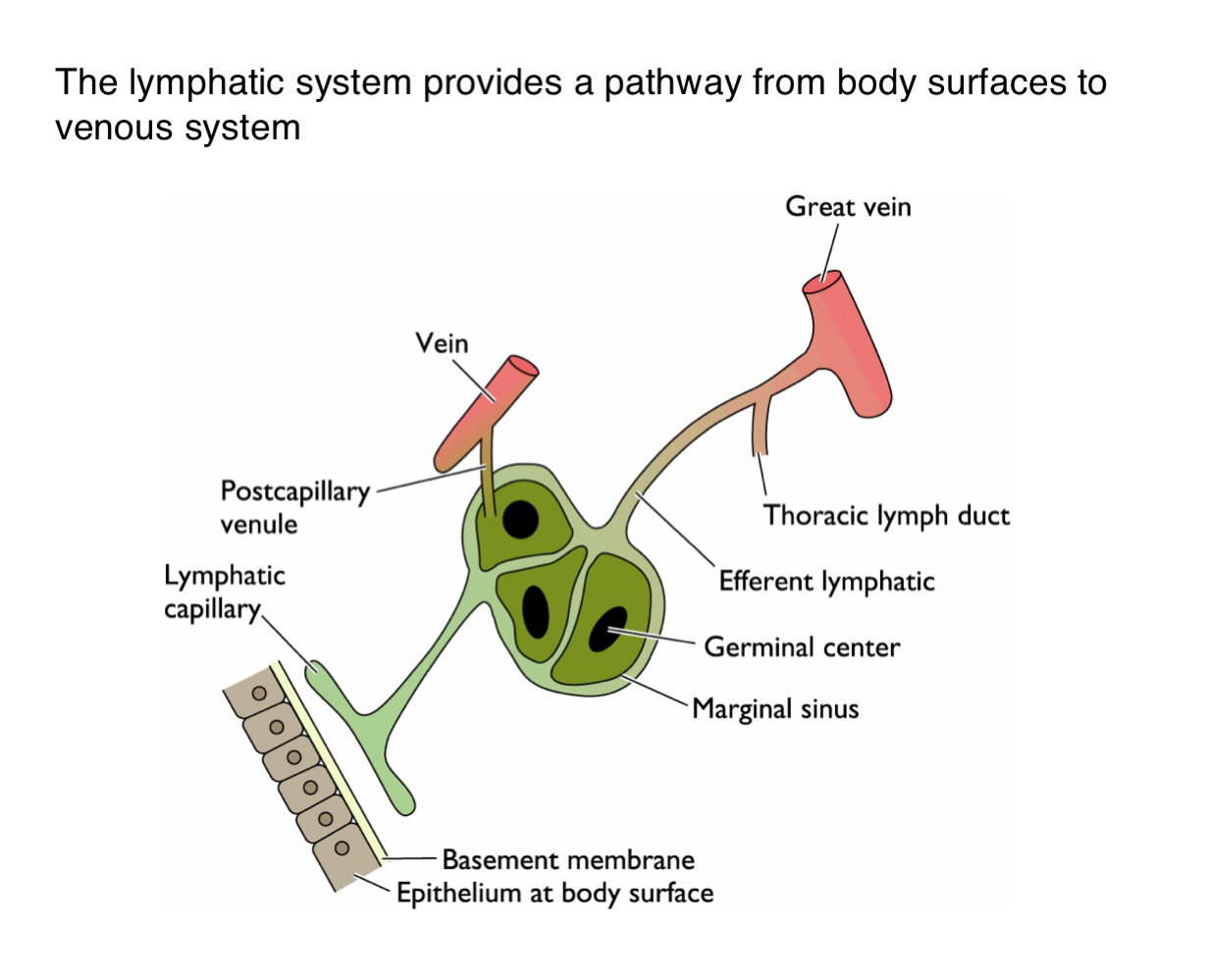
How do viruses transition from lymphatics to the bloodstream?
After amplification in the lymph nodes, viruses enter larger lymphatic vessels (e.g. thoracic duct and eventually drain into the veins)
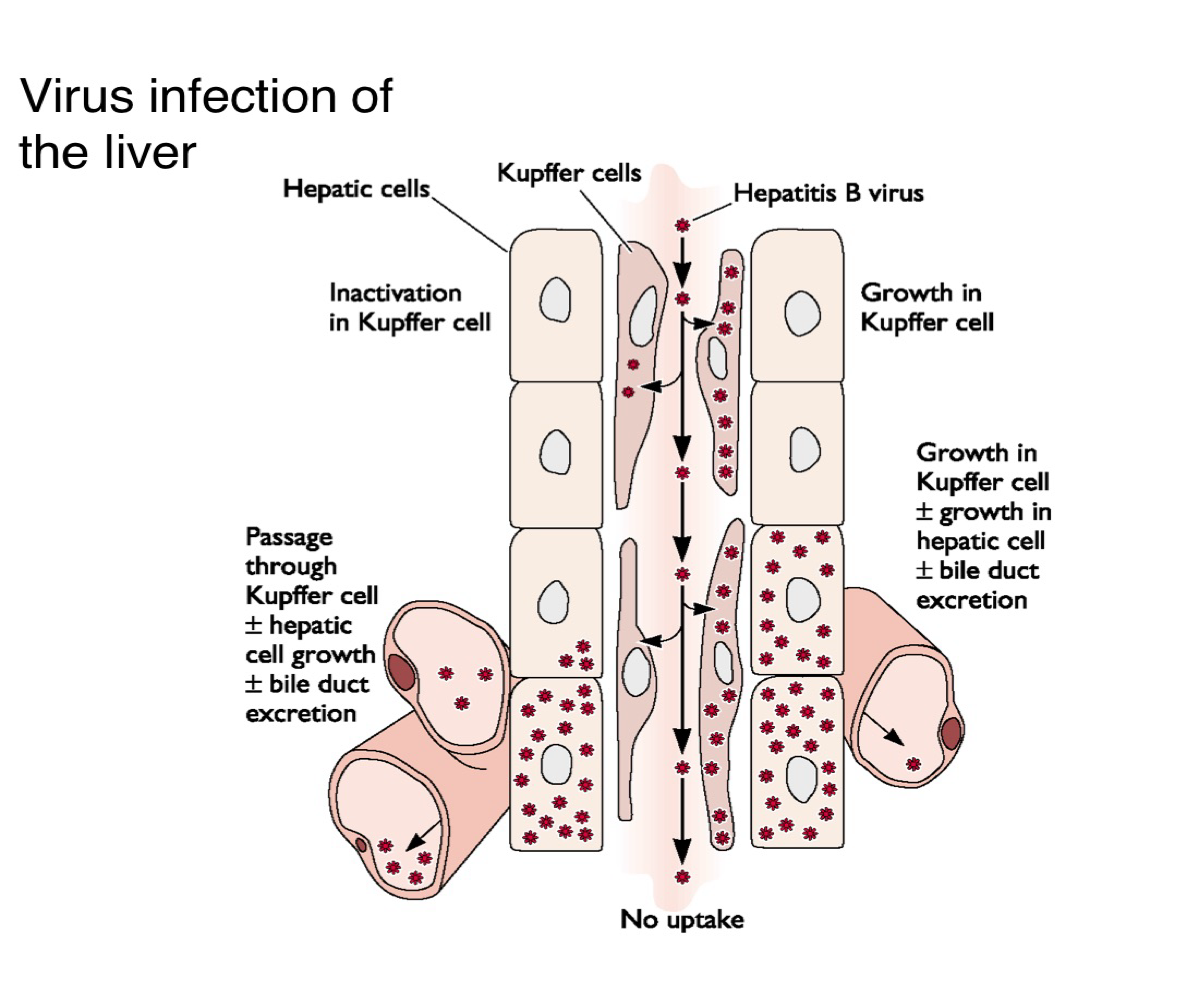
What are Kupffer cells and what is their role in liver viral infection?
Are liver-resident macrophages that can inactivate viruses or allow them to pass through to infect hepatocytes
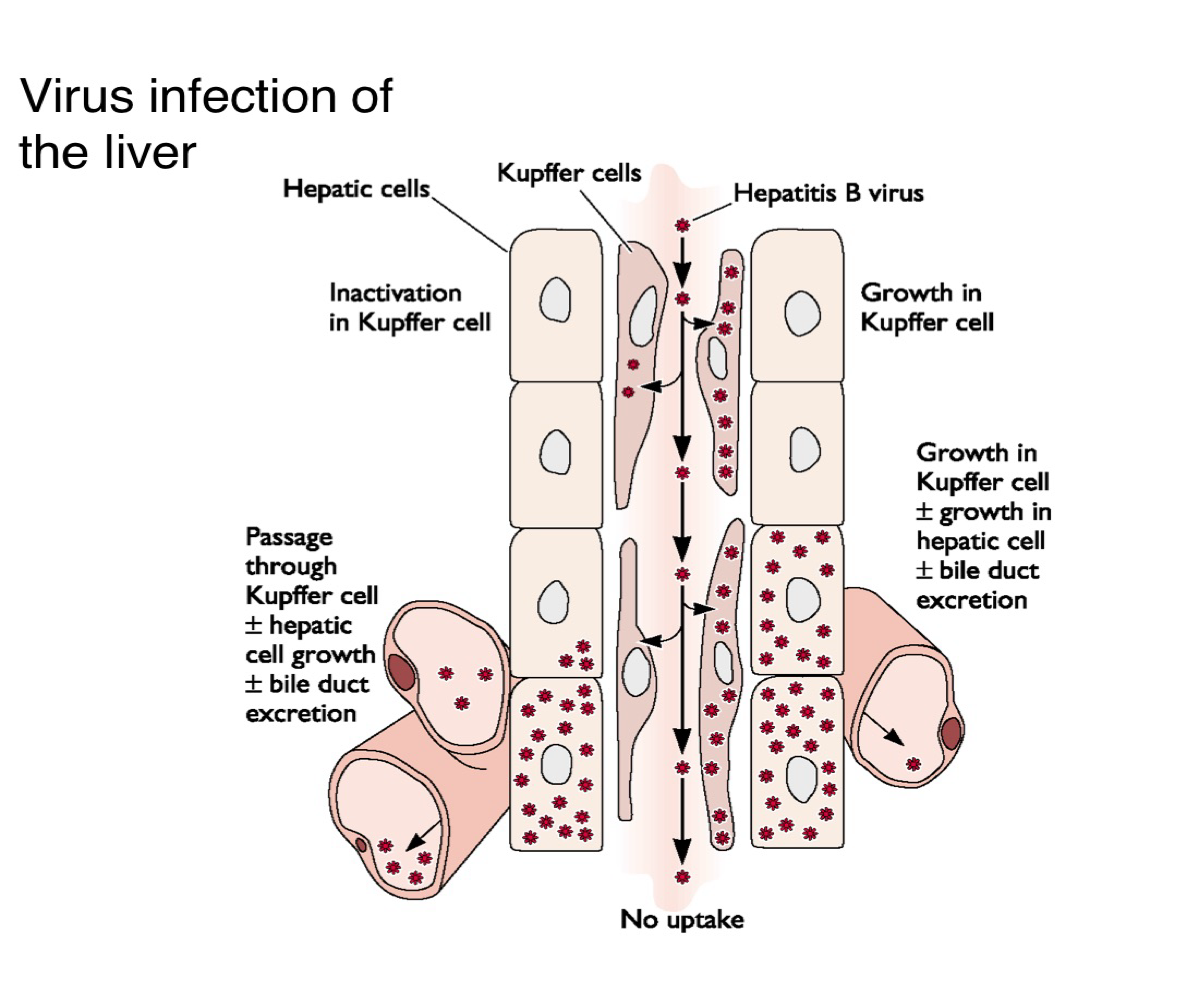
Descrive two possible outcomes when a virus encounters Kupffer cells in the liver?
The virus is inactivated and cleared
It passes through, infects hepatocytes, and contributes to bile duct excretion and viral replication
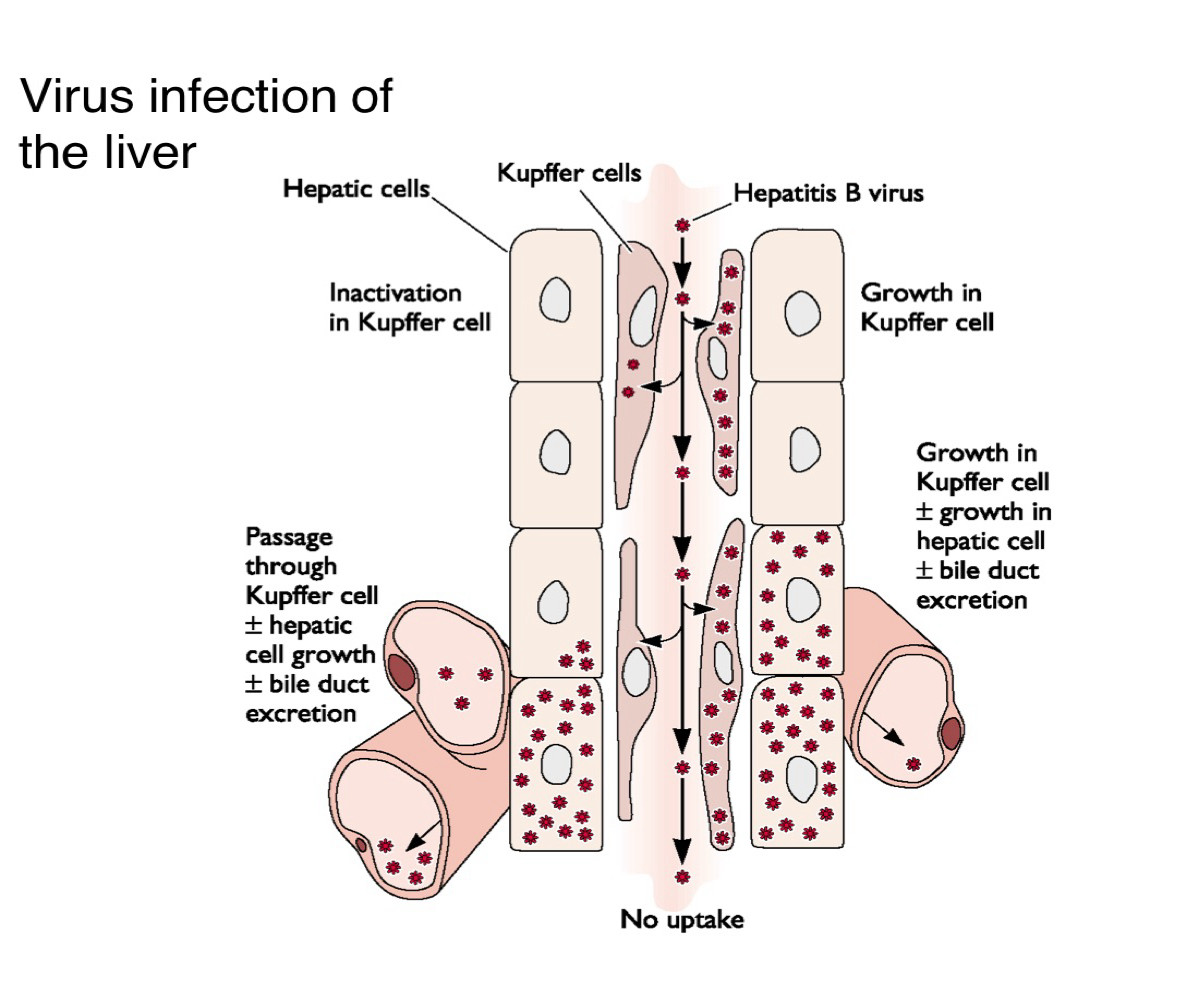
How does hepatitis B virus spread within the liver?
It may replicate in the Kupffer cells and/or hepatocytes, leading to liver pathology and viral shedding into the bile
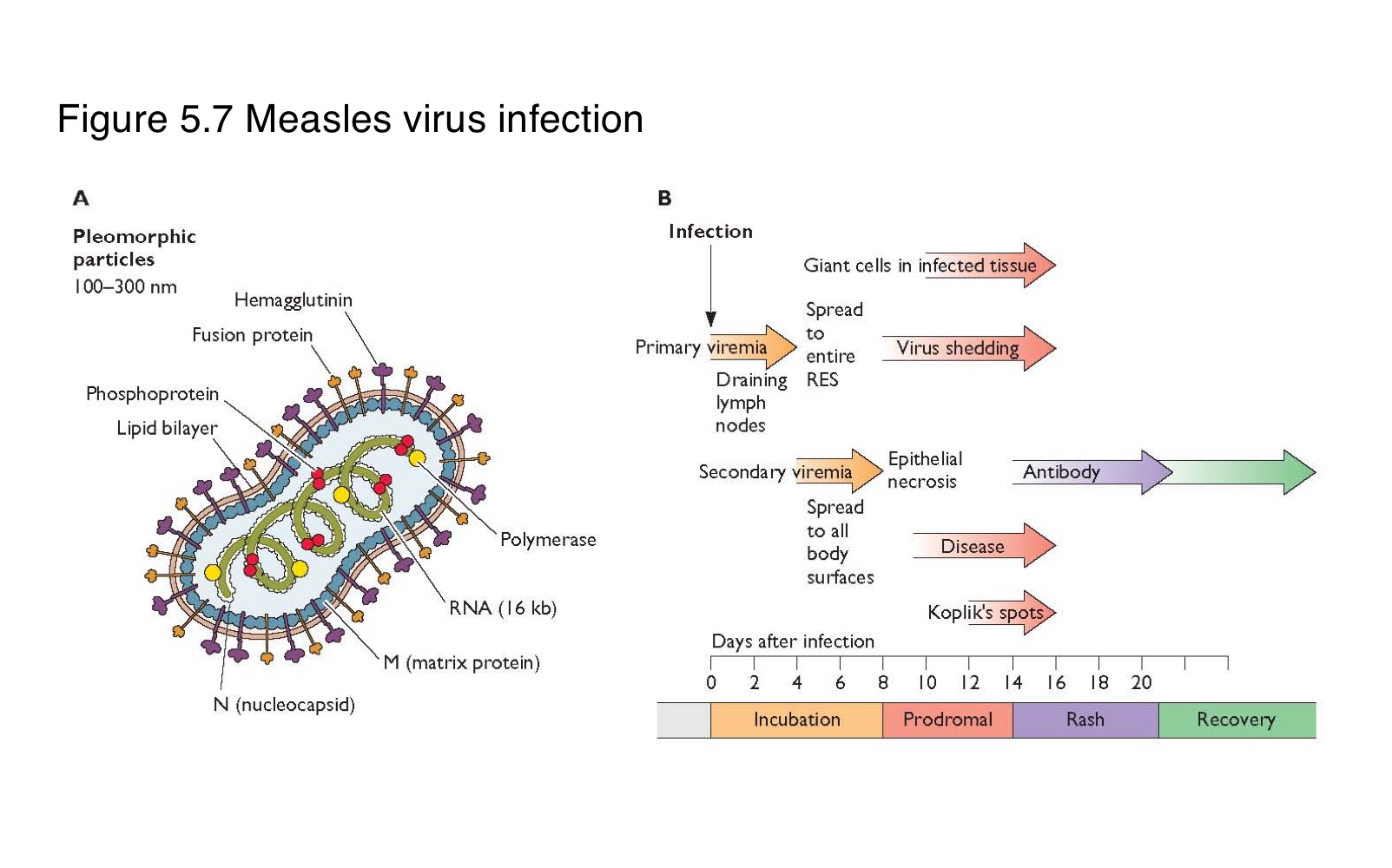
Where does measles virus first replicate after entering the body?
In the epithelial cells of the respiratory tract
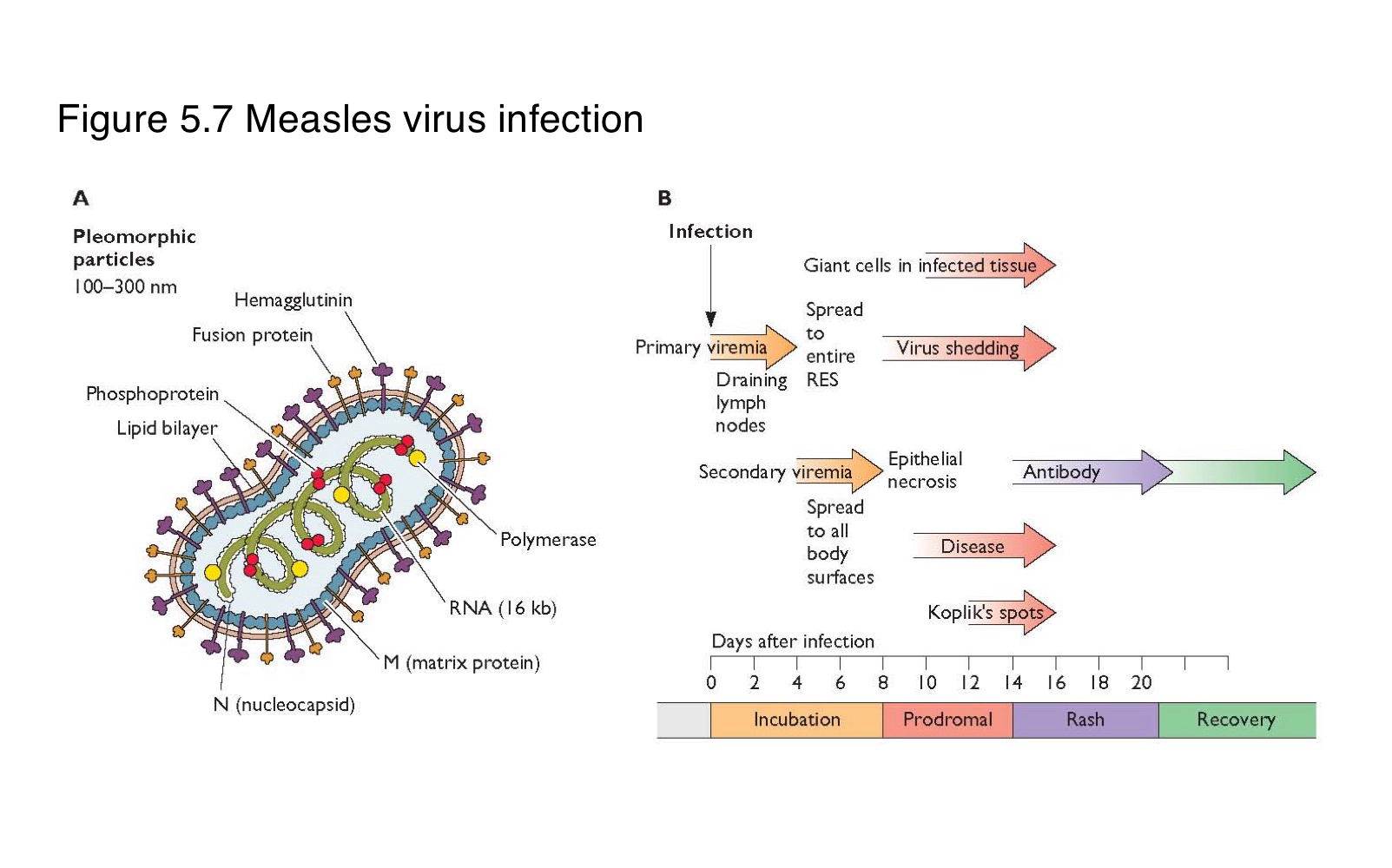
What is the sequence of events following primary replication in a measles infection?
The virus enters local lymph nodes → causes primary viremia → infects reticuloendothelial cells → leads to secondary viremia → spreads to skin and other organs
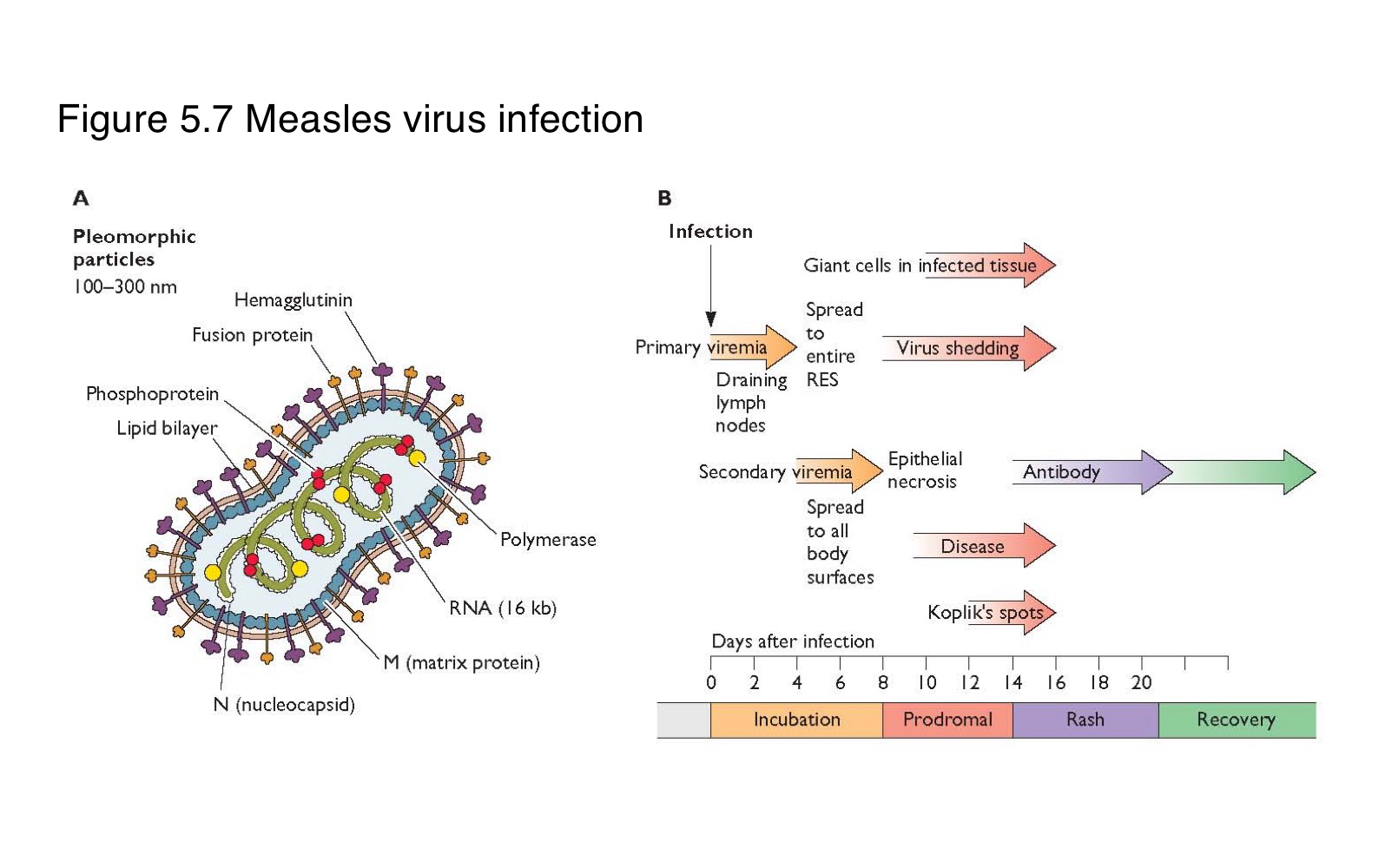
What is the difference between primary and secondary viremia in measles infection?
spreads the virus from the site of entry to lymphoid organs
disseminates it widely to the skin and mucosa
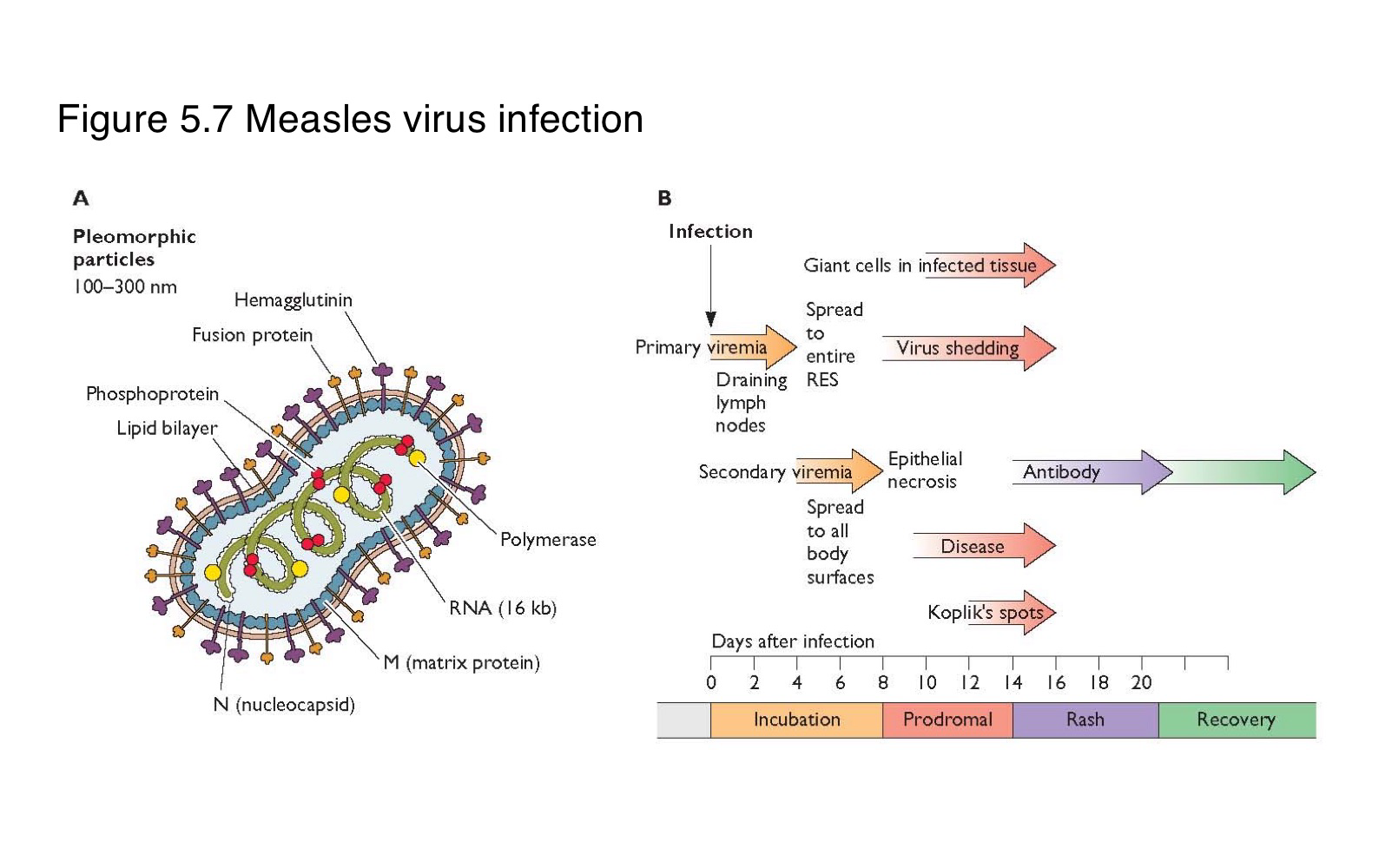
What clinical feature marks the systemic spread of measles virus?
The appearance of skin rash, typically during the secondary viremia phase
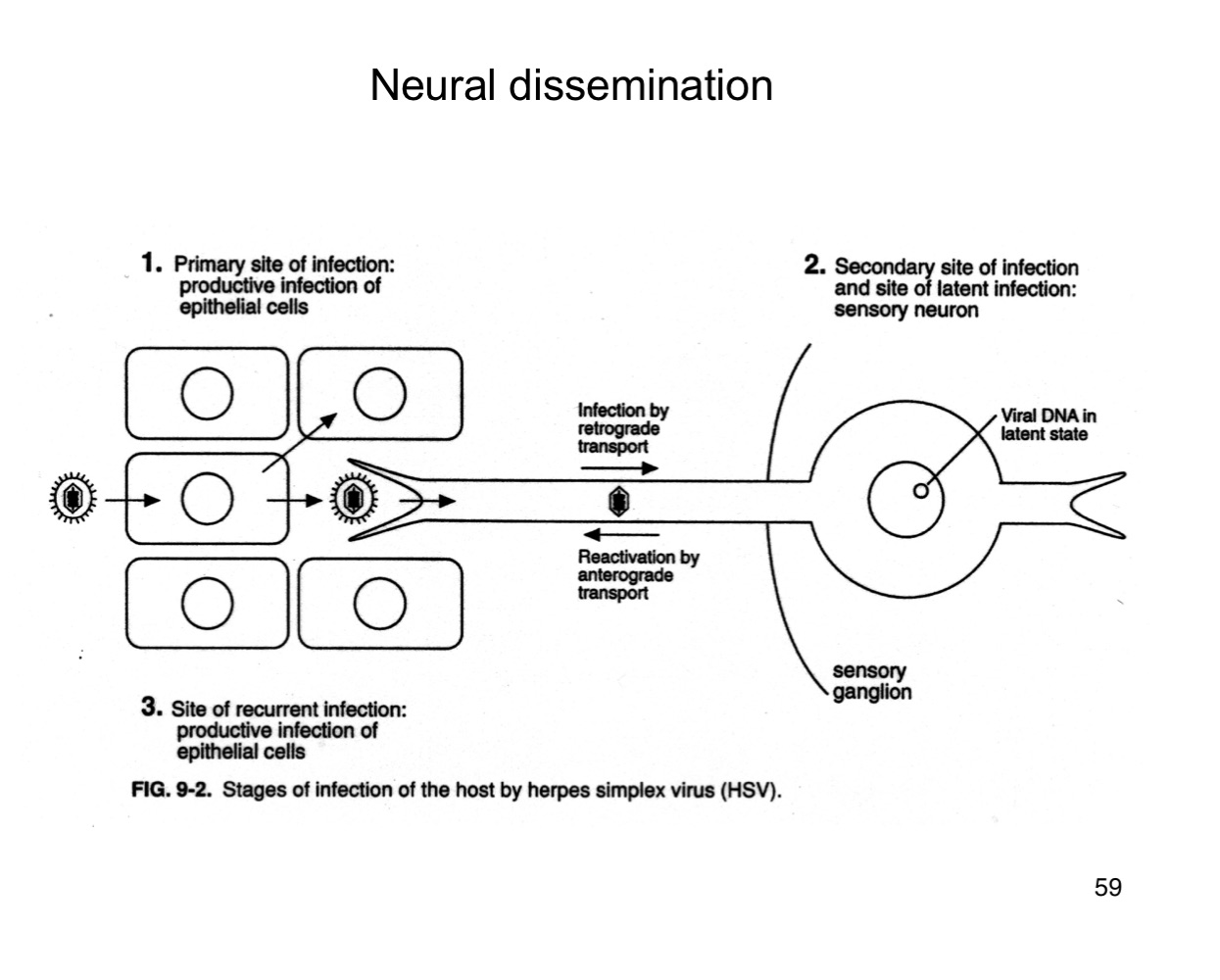
How does herpes simplex virus (HSV) reach the nervous system?
It infects sensory neurons at the primary site and travels retrograde to the sensory ganglia.
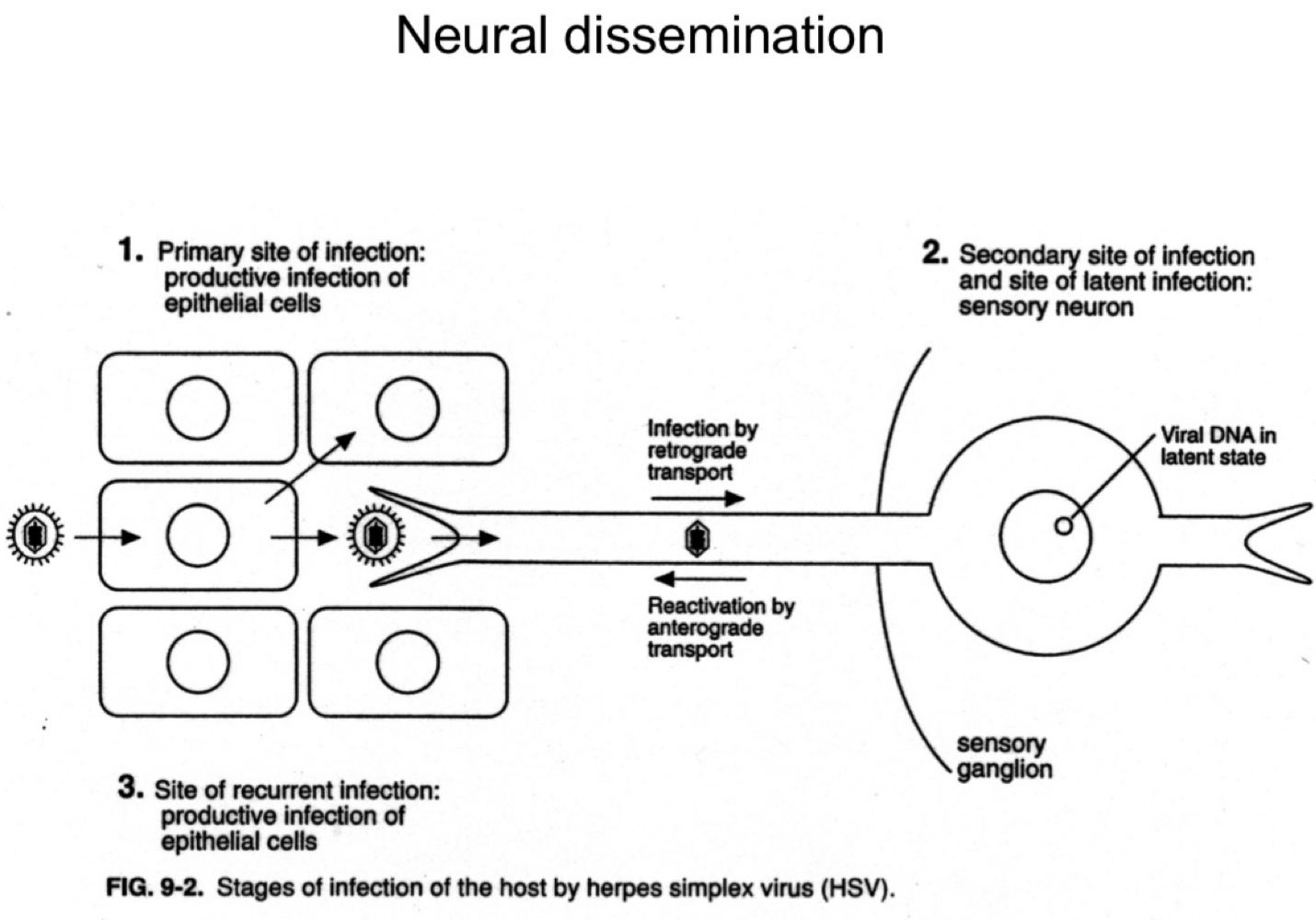
What happens to HSV after it reaches the sensory ganglia?
The virus establishes latency in the neuronal cell bodies.
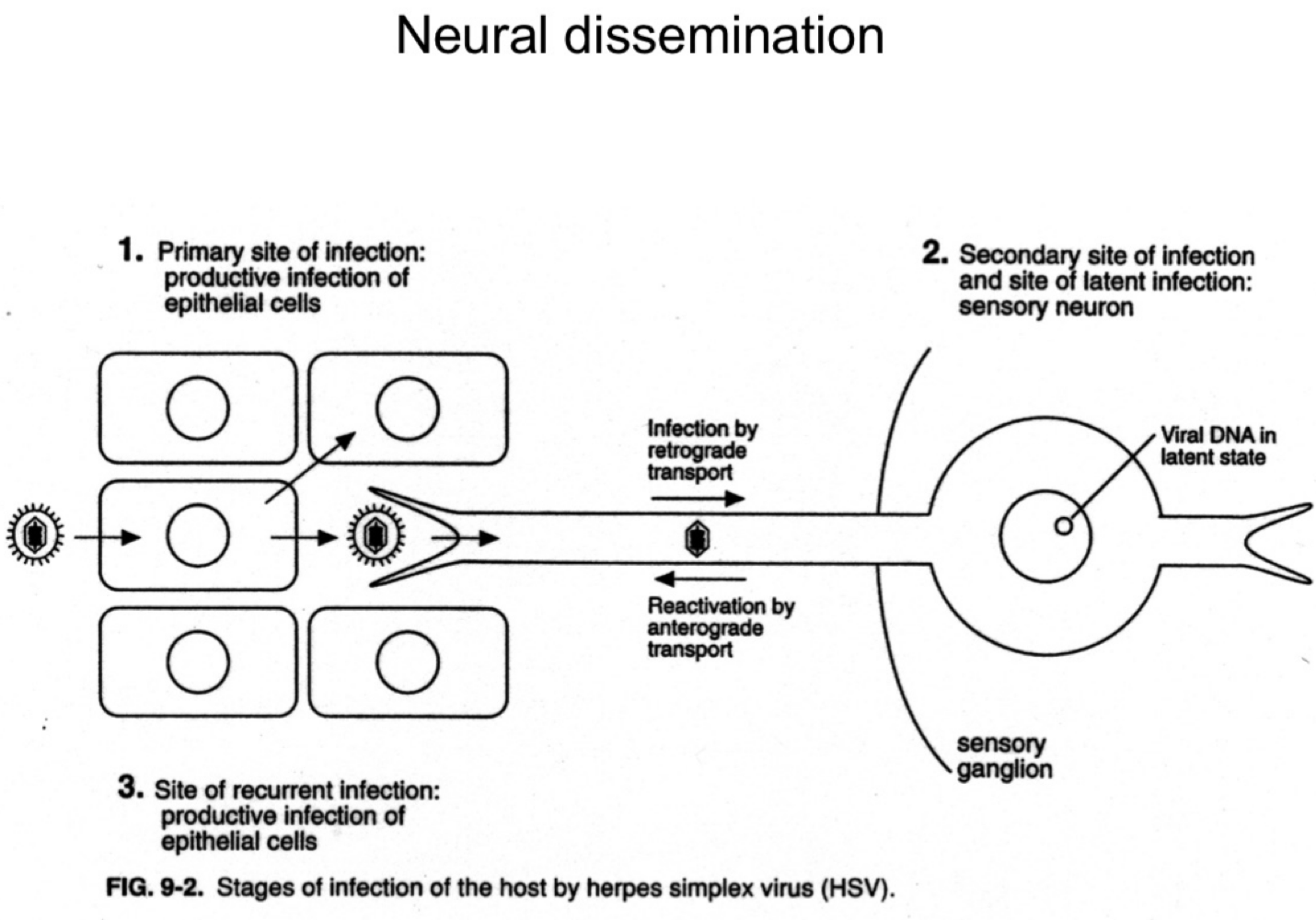
How is HSV reactivated in the body?
Upon reactivation (e.g., stress, immunosuppression), HSV travels anterograde back down the neuron to epithelial sites, causing recurrent lesions.
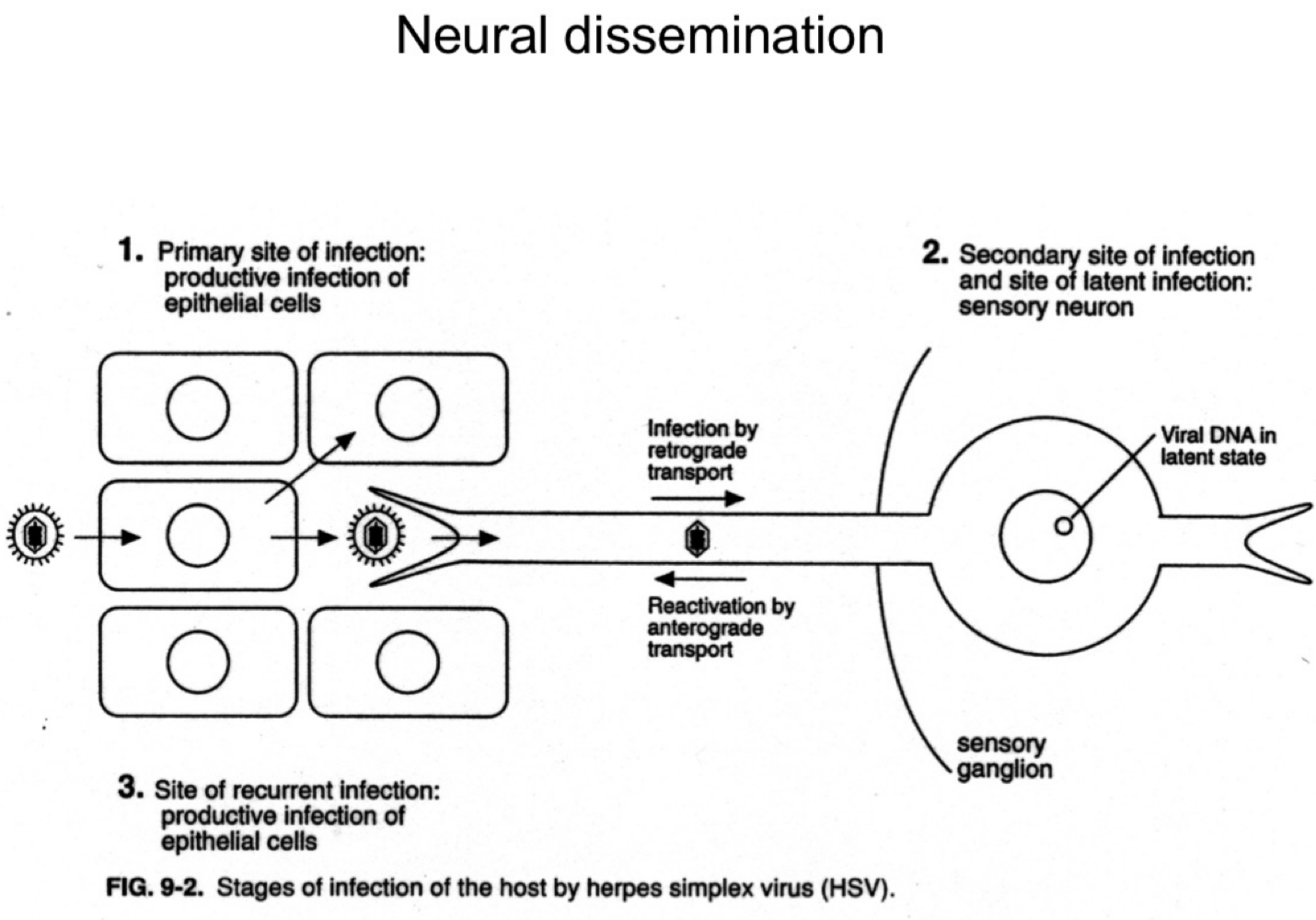
What are the three phases of HSV infection relative to the nervous system?
Primary epithelial infection
Retrograde transport to ganglia and latency
Anterograde transport during reactivation.
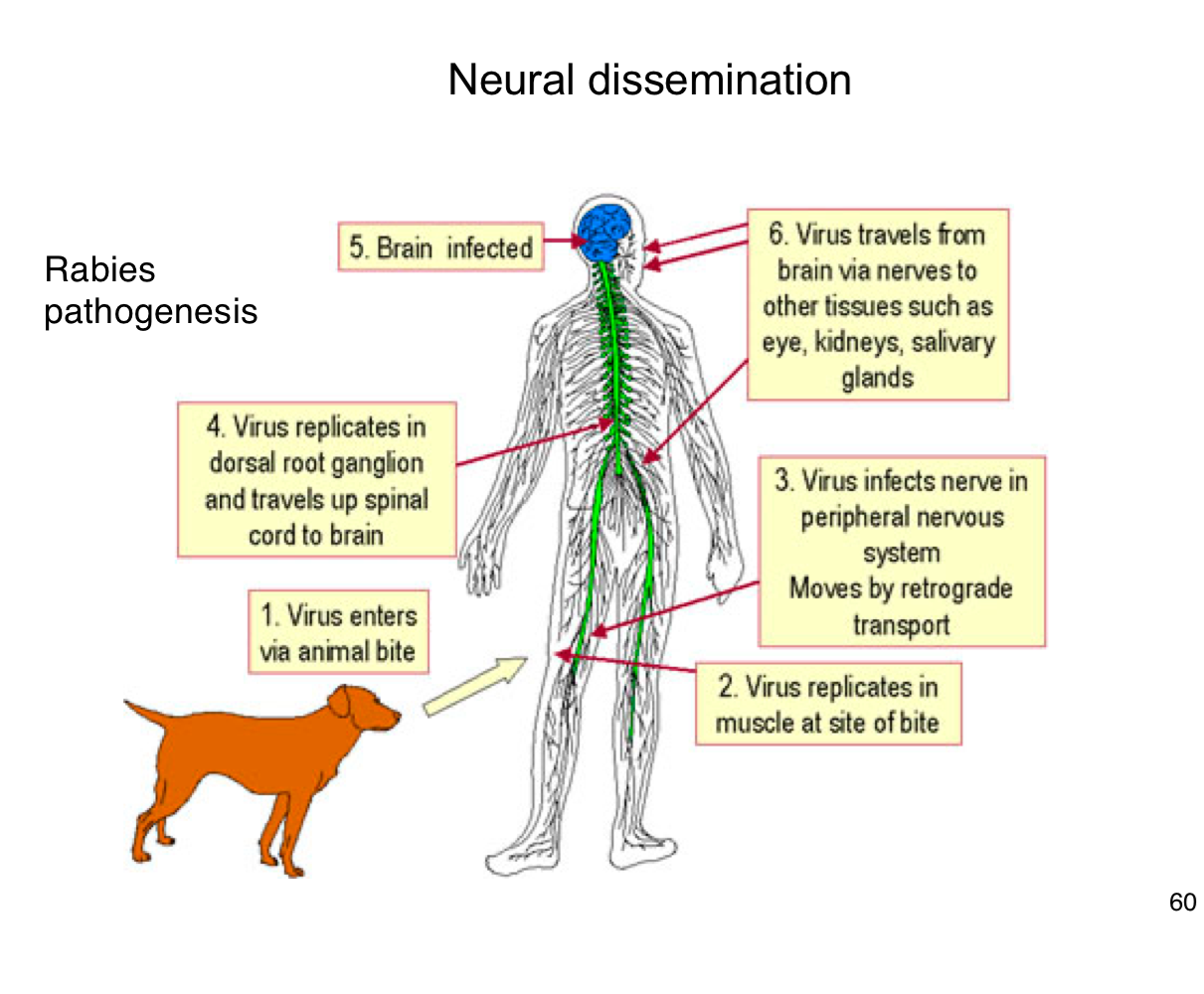
How does rabies virus typically enter the human body?
Through the bite of an infected animal, introducing the virus into muscle tissue.
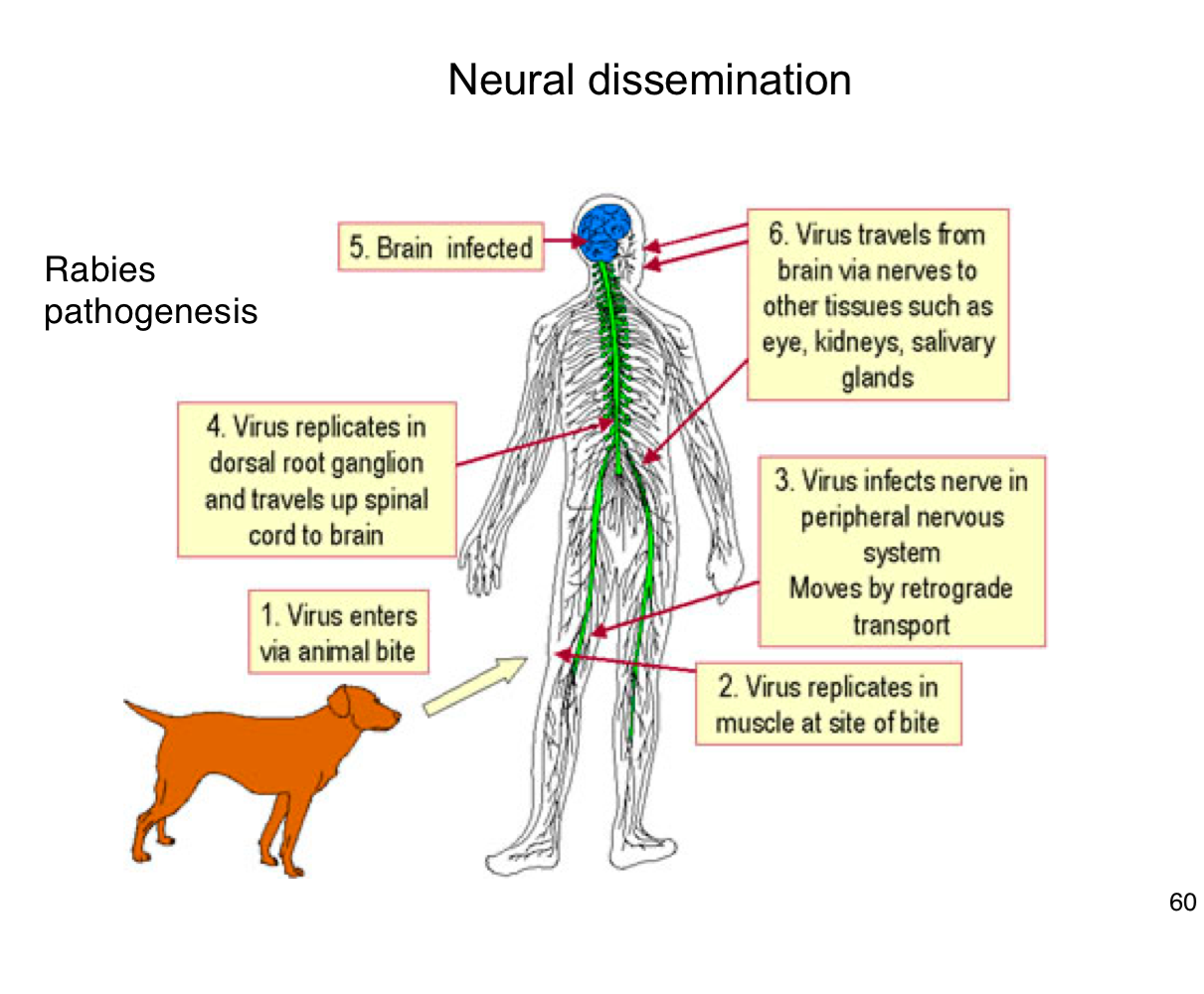
Where does rabies virus initially replicate in the body?
In muscle cells at the site of the bite
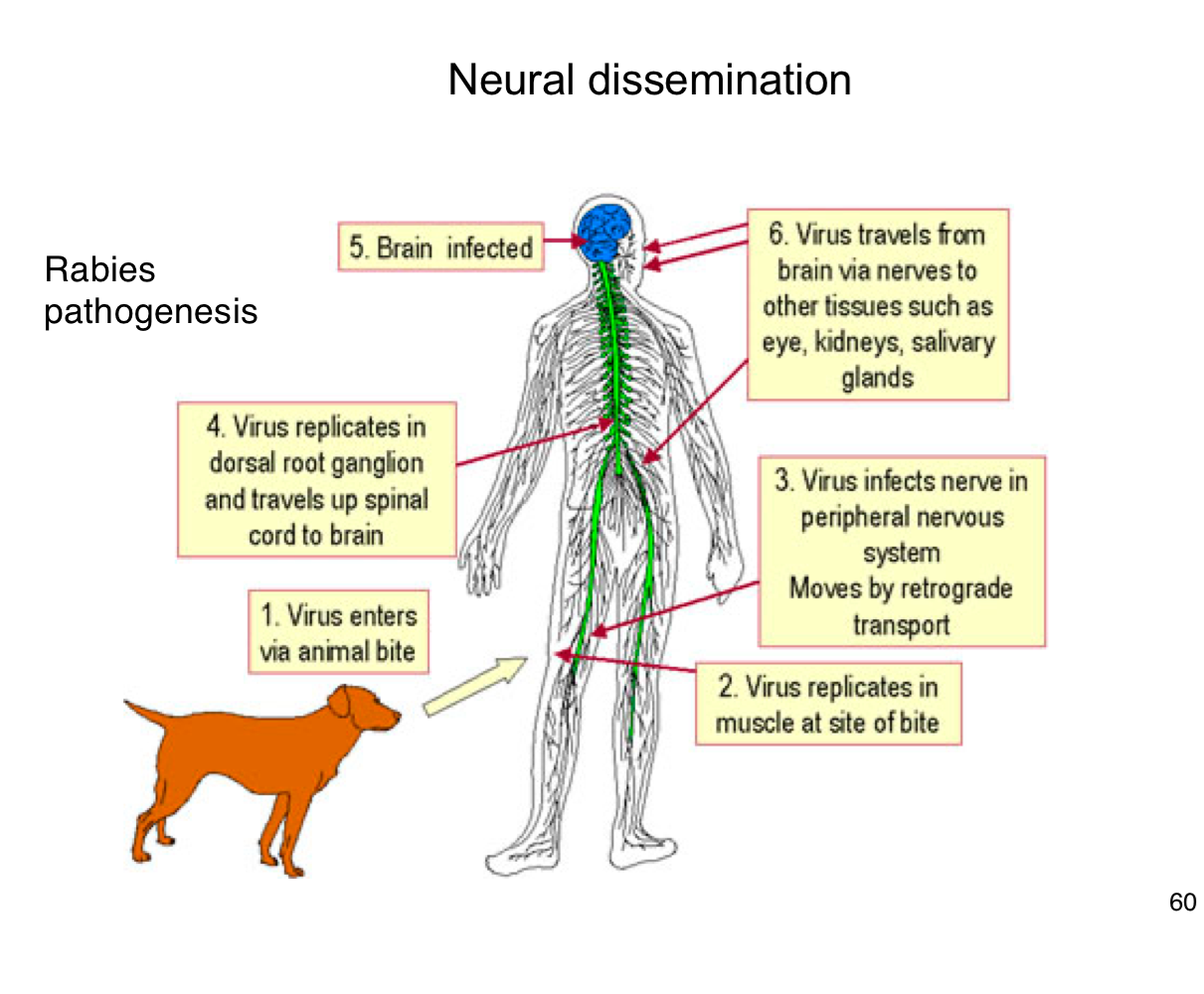
How does rabies reach the central nervous system (CNS)?
It infects peripheral nerves and moves by retrograde axonal transport to the spinal cord and brain
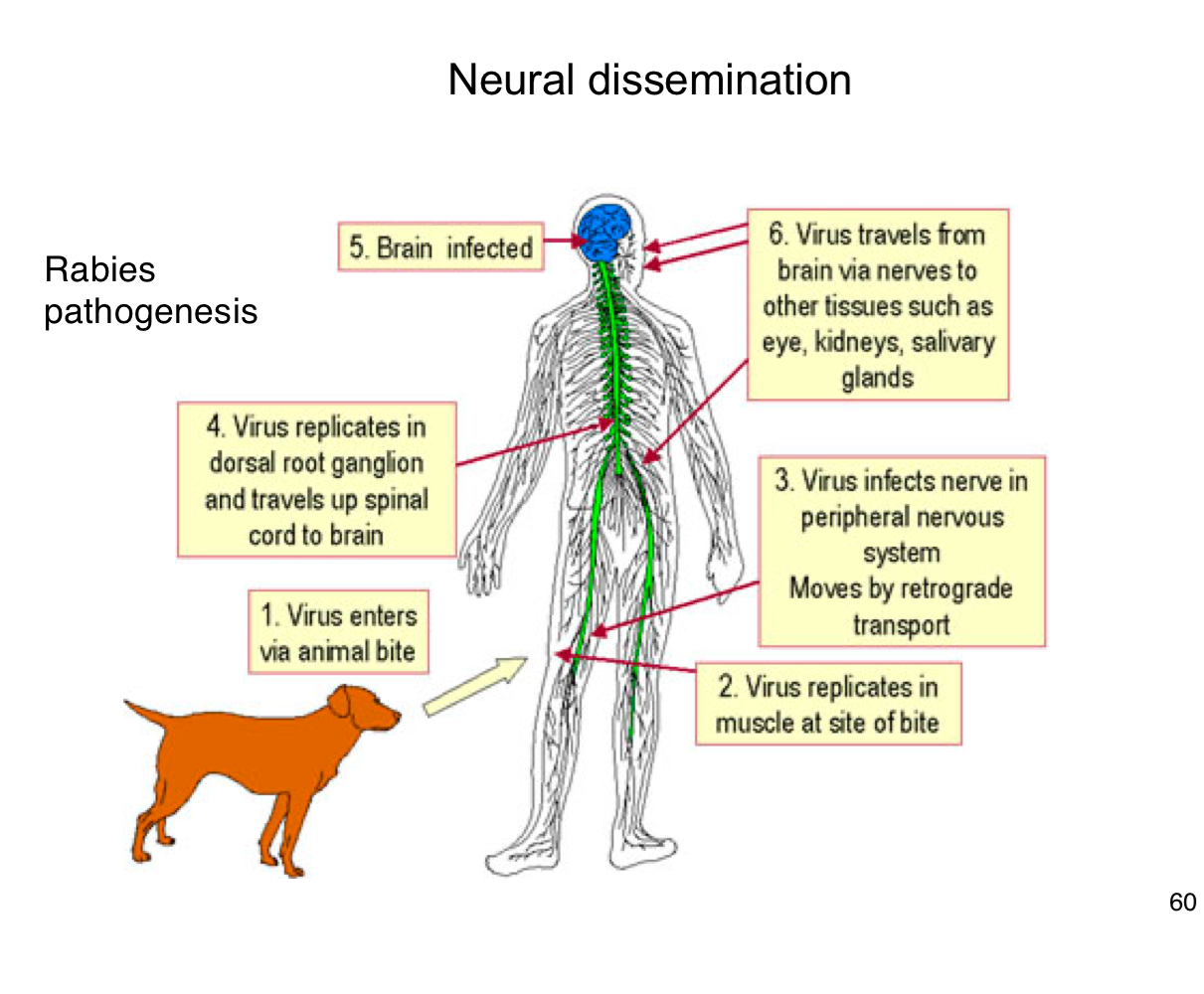
What happens after rabies virus reaches the CNS?
It replicates in the brain, leading to encephalitis and severe neurological symptoms.
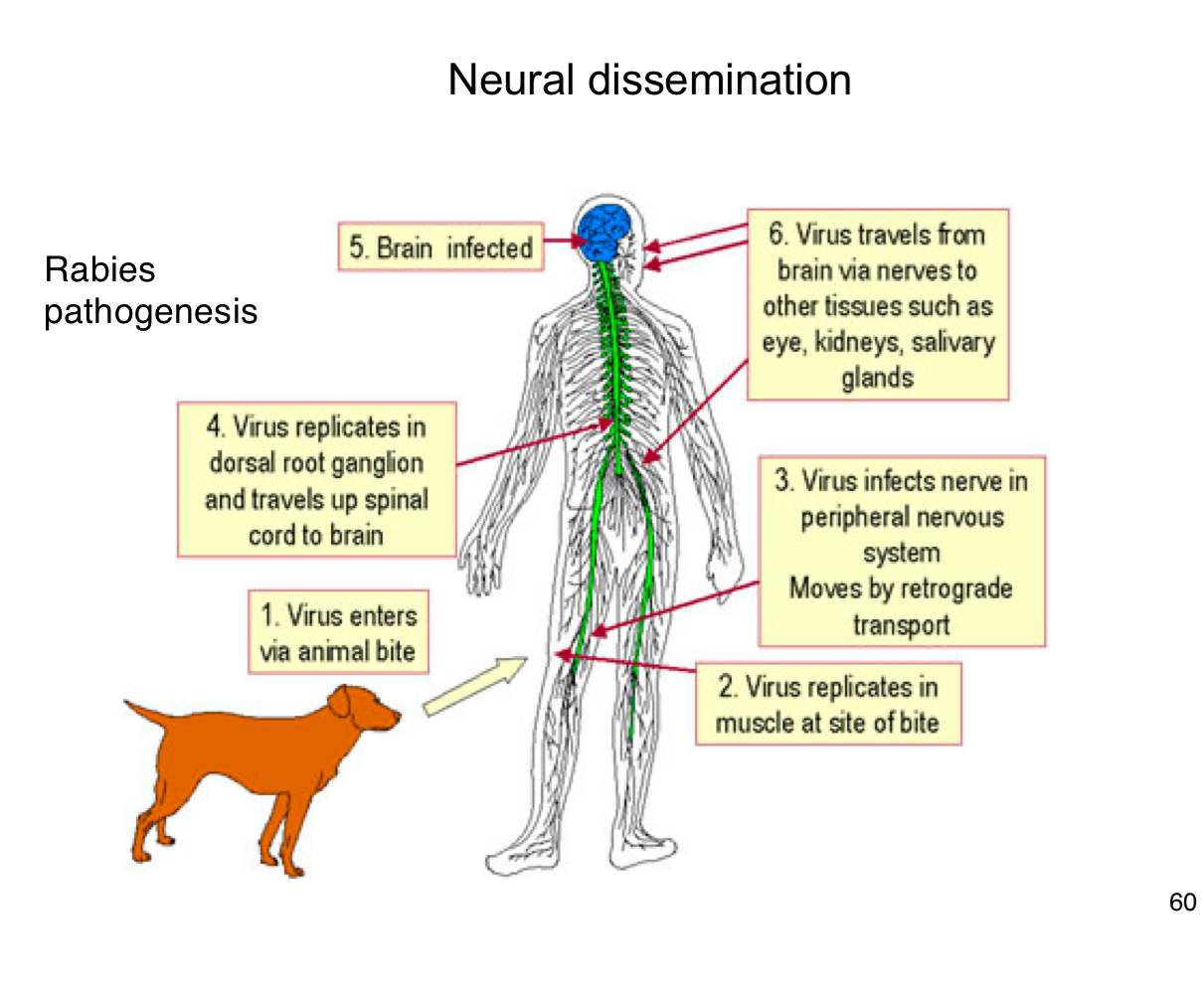
After infecting the brain, how does rabies spread to other tissues?
It travels along peripheral nerves to tissues like the eyes, salivary glands, and kidneys.
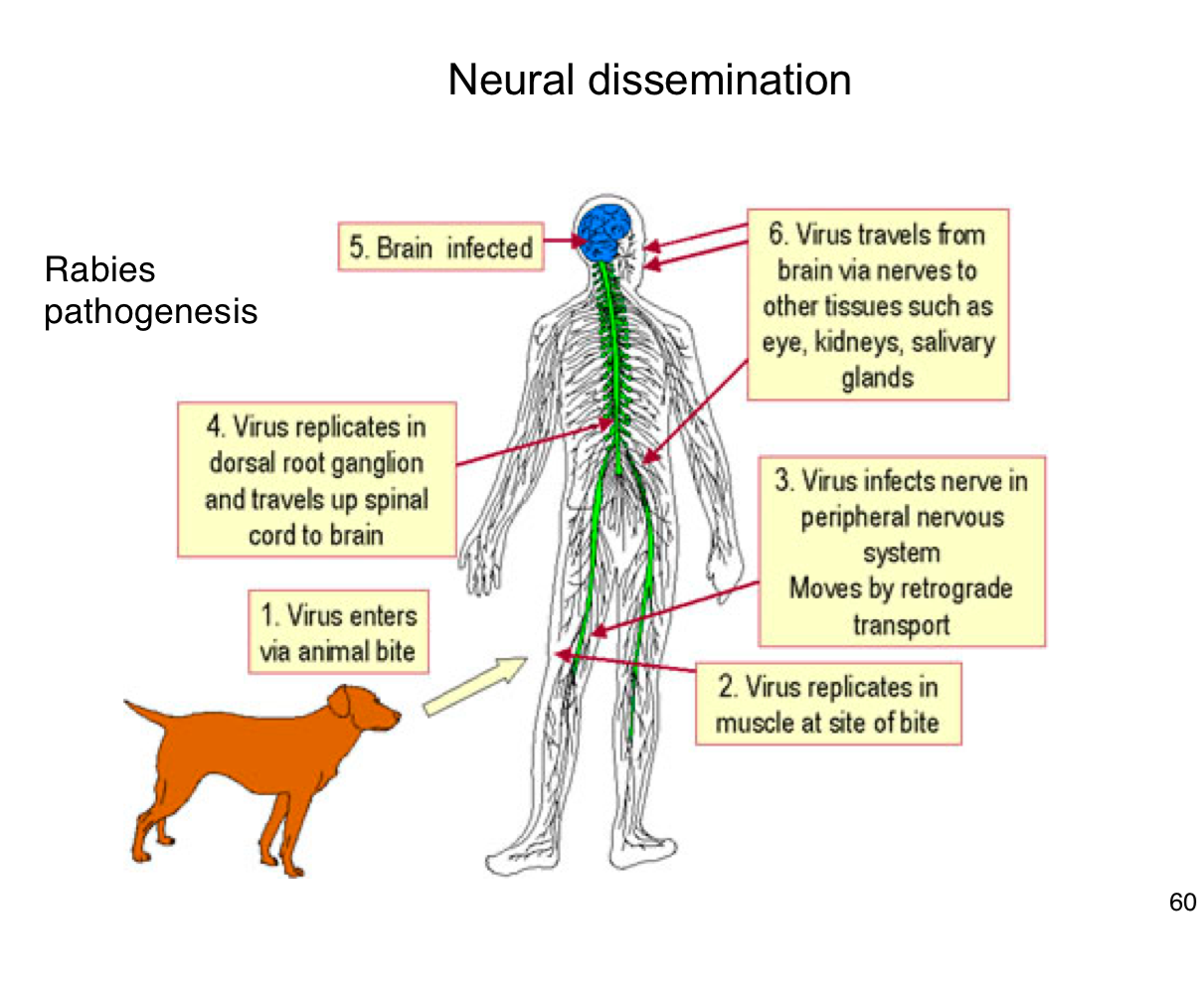
Why is early vaccination crucial after potential rabies exposure?
Because once the virus enters the nervous system, it becomes much harder to treat and almost always leads to death if untreated
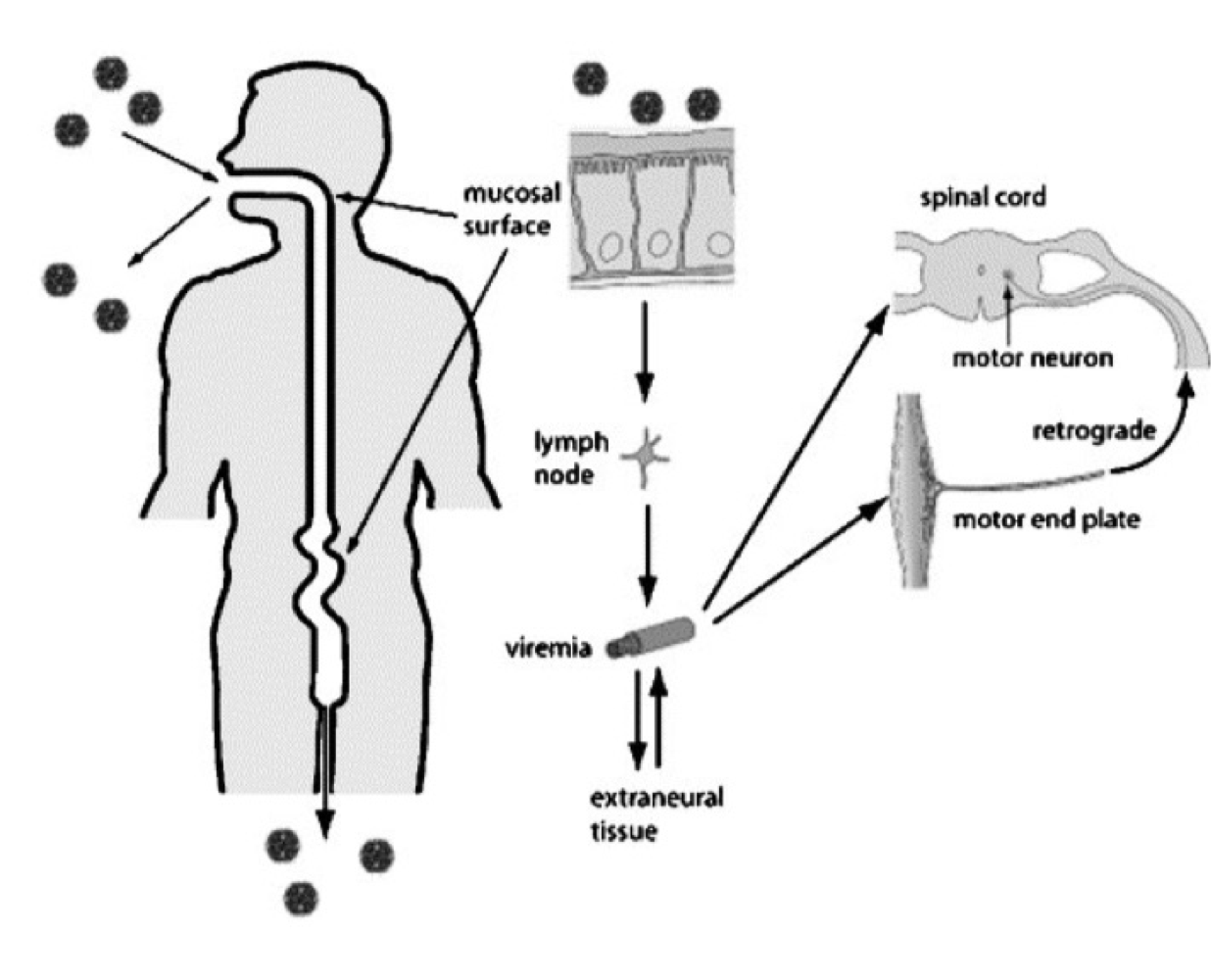
How is poliovirus typically transmitted between individuals?
Primarily via the fecal-oral route.
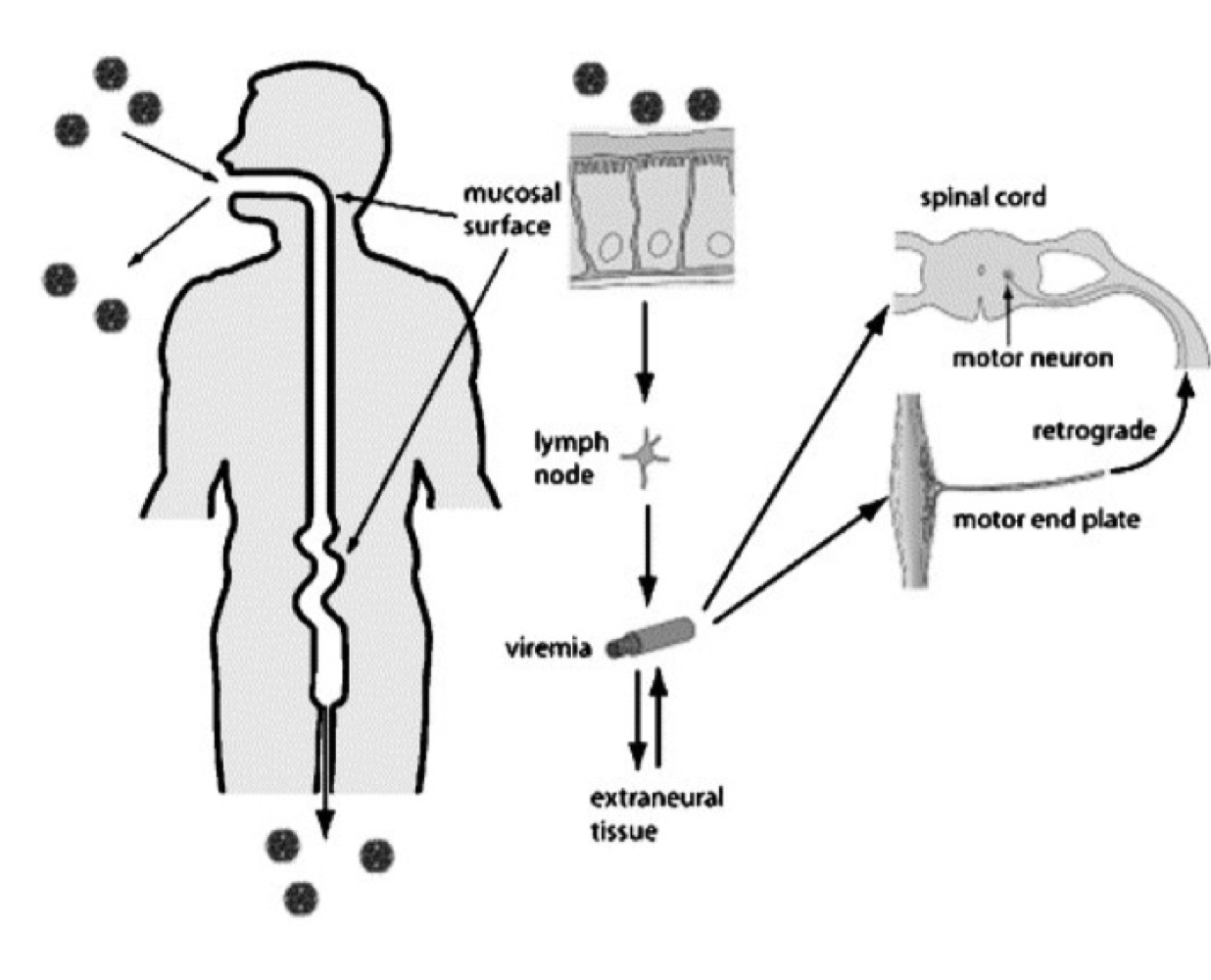
Where does poliovirus first replicate in the human body?
In epithelial cells of the oropharynx and intestinal mucosa