Chemistry - Structure and Bonding + periodic table
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
Describe Antoine Lavoisier’s periodic table (1789)
Split all ‘elements’ into 4 categories: acid making, gas-like, metallic, and earthy. These included light, heat, and lime (calcium oxide)
What had not yet been discovered when early scientists tried to create the periodic table
Protons, electron, neutrons
Describe John Newlands periodic table (1864)
He organised the elements in rows of 8 (Law of Octaves), stating that elements in a column all had similar properties. Many of the elements were in the same space as others, and, as some had not yet been discovered, a lot of them that were put in the same group did not actually share properties
Disadvantages of Newland’s table
Two elements may share a space (eg. Cobalt and Nickel)
Some elements in the same group had very different properties
No clear division between metals and non - metals
Newland didn’t allow spaces for new elements
How did Mendeleev’s table improve on Lavoisier’s and Newland’s
Included elements which had not yet been discovered
Organised elements in order of their atomic mass - but with some exceptions to ensure that they matched the properties of their group
Separates Metals and Non metals
Why did scientists not accept Mendleev’s table immediately
Did not see evidence for his predictions of eka- aluminium
Left gaps = table is incomplete
Some elements not in order of RAM means that the rule is not universal
Why did the discovery of Galium convince other scientists that Mendeleev was correct
His prediction was very accurate - especially of its atomic mass. It perfectly filled the gap in group 3
The discovery of what explained why the rule of RAM could not always be followed
Isotopes
Where are the alkaline metals
Group one
Metals are elements that react to form ions
positive
What to the alkaline metals form when they reacts with water
An alkali (shock)
What is more reactive: Lithium or Francium
Francium
Why is Francium more reactive than Lithium
It has more outer shells ->outer most shell is further away from nucleus -> forces of attraction holding it in place are weaker -> can react more easily with other elements bc it needs to lose it’s electron
What can be observed when lithium reacts with oxygen
Burns with a red flame, giving a white solid
What can be observed when lithium reacts with water
Sizzling, steam, vibrating, moving side to side across the surface of the water, gas produced. Water turns purple with a universal indicator
What can be observed when lithium reacts with chlorine
Vigorous reaction, white solid formed which dissolves in water to give a neutral solution
What can be observed when sodium reacts with oxygen
Burns with a golden yellow colour to give a white solid
What can be observed when sodium reacts with water
Fizzing, burning, cracking, bright orange flame, violent reaction. Water turns purple with a universal indicator
What can be observed when sodium reacts with chlorine
Burns intense yellow/orange colour. White smoke produced. Exothermic reaction. Neutral solution formed
What can be observed when potassium reacts with oxygen
Burns with a lilac flame to give a white solid
What can be observed when potassium reacts with water
Fizzing (effervesance), crackling, lilac flame produced. Water turns purple with a universal indicator
What can be observed when potassium reacts with chlorine
Large bright lilac flame, suddenly pops and spontaneously combusts, potassium deposits can be seen
Why is it unsafe to use rubidium, caesium, and francium in a reaction in a lab
It reacts violently with water, and oxygen, so causes a large explosion
What is formed when an alkali metal reacts with water
Metal hydroxide and hydrogen
What is formed when an alkali metal reacts with oxygen
Metal oxide
What is formed when an alkali metal reacts with chlorine
Metal chloride
Where are the halogens
Group 7
What do the halogens form when they react with water
A salt
What is more reactive, tennessine or fluorine
fluorine
Why is fluorine more reactive than than tennessine
The halogens need to gain an electron -> fluorine’s outer shell is closer to its nucleus, so is under greater attractive force -> this means that its forces of attraction will be able to act more strongly on another atom’s electron, as there is a shorter distance -> it reacts and gains an electron more easily
Describe fluorine
Poisonous yellow gas. Very reactive
Describe chlorine
Poisonous green gas. Reactive, but less than fluorine
Describe bromine
Red/brown poisonous volatile liquid
Describe iodine
Grey solid, can form poisonous purple vapours, but is also an antiseptic
Halogens always come in____
Diatomic molecules, bonded covalently (pairs)
What do halogens form with non metals
Covalent bonds
What do halogens form with metals
Ionic bonds
When you go down the halogens, the melting and boiling point increases/decreases
increases
When you go down the halogens, the reactivity increases/decreases
decreases
What is a halogen called when it forms an ionic compound
Halide
What is a displacement reaction
A reaction in which a more reactive element displaces another one in a compound
If chlorine is added to potassium bromide, what happens/what is formed
Potassium chloride + bromine
If iodine is added to potassium fluoride, what happens/what is formed
No change
What colour are halide salts
colourless
What colour is bromine water
orange/yellow
What colour is chlorine water
colourless
What colour is iodine water
brown
When you mix (insert halogen) water with a salt, what happens
Colour change
What do you observe when you mix potassium bromide with chlorine in water and why do you observe this
Solution trunks from colourless to orange/yellow.
Before, chlorine was present (which is colourless), but the chlorine displaces the bromine, and the bromine is orange/yellow
What colour is chlorine when dissolved in hexane
Pale green
What colour is bromine when dissolved in hexane
orange
What colour is iodine when dissolved in hexane
purple
What are group 0/group 8 elements called
Noble gases
True or False: Noble gases are very reactive?
False - Noble gases are unreactive
Why are noble gases unreactive?
They have a full outer shell of electrons, so don’t need to gain or lose any to be stable. This means that they don’t react with other elements
Does the melting and boiling point elements increase or decrease as you move down the group and why
Increase because, as you move down the group, the atoms have more electrons. This means that the intermolecular forces of attraction between molecules are stronger and less easily overcome, so it takes more energy to break them.
Alkali metal or transition metal: low melting points
Alkali metals
Alkali metal or transition metal: high melting point
Transition metals
Alkali metal or transition metal: not very reactive
Transition metals
Alkali metal or transition metal: very reactive
Alkali metals
Alkali metal or transition metal: can be used as a catalyst
Transition metals
Alkali metal or transition metal: coloured compounds
Transition metals
Alkali metal or transition metal: white compounds
Alkali metals
Alkali metal or transition metal: ionic compounds formed when reacted with non metals
Alkali metals + Transition metals
Alkali metal or transition metal: good thermal and electrical conductors
Transition metals + alkali metals
Alkali metal or transition metal: strong and hard
Transition metals
Alkali metal or transition metal: soft and can be cut with a knife
Alkali metals
Alkali metal or transition metal: high density
Transition metals
Alkali metal or transition metal: low density, some float on water
Alkali metals
Alkali metal or transition metal: form an alkali solution with water
Alkali metals
Alkali metal or transition metal: react with water releasing a hydrogen gas
Alkali metals
Alkali metal or transition metal: form 1+ ions
Alkali metals
Alkali metal or transition metal: can form different ions
Transition metals
Describe transition metals (compounds and ions, uses, density, melting point)
Coloured compounds, different ions formed, used as catalysts, jewellery etc, high density, high melting point
Solid to liquid
melting
Liquid to gas
evaporation
Gas to liquid
condensation
Liquid to solid
solidifying/feezing
Solid to gas
deposition
Gas to solid
sublimation
What does a graph showing ice/water/steam being heated look like (image on other side)
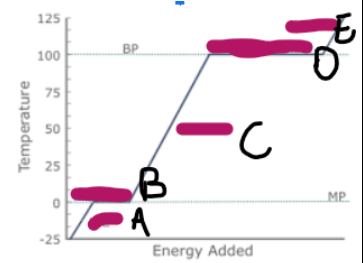
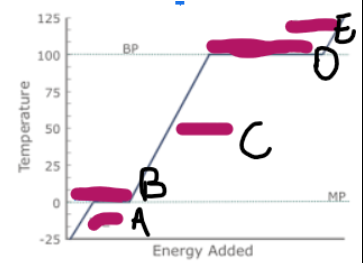
What is happening at A
Ice is being heated, and the particles are vibrating faster
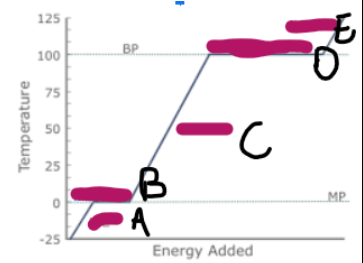
What is happening at B
Melting
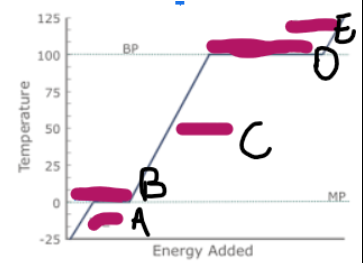
What is happening at C
Water is being heated, and the particles are moving and vibrating more vigorously
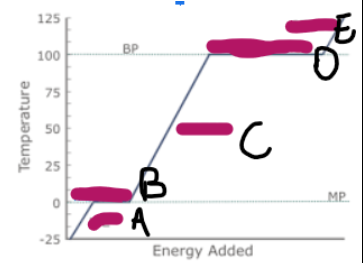
What is happening at D
Evaporation
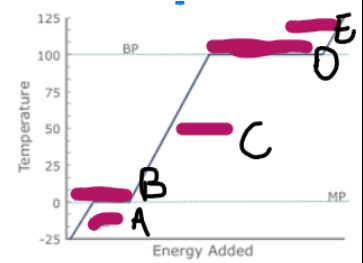
What is happening at E
Steam is being heated, and as it has more kinetic energy, is moving faster, making the particles spread out, and the volume increase
What is the structure of a diamond
Giant lattice. Made up of carbon atoms, which each bond to 4 other carbons
Why do diamonds have a high melting point
Giant lattice structure - each c bonds to 4 others
Covalent bonds between them are strong
Lots of energy is needed to break these bonds
Why are diamonds very strong
Giant lattice structure - each c bonds to 4 others
Covalent bonds between them are strong
It is very difficult to move any carbon atom out of place
Why do diamonds no conduct electricity
Giant lattice structure - each c bonds to 4 others
No electrons are free to carry charge
Diamonds cannot conduct electricity
What are the uses of diamonds
Gemstones
Abrasures
Rock drills and saws
Cut glass
What is the structure of sand
Giant Lattice. Made up of silicon (which bonds to 4 atoms) and oxygen (which bonds to 2). Looks similar to diamond except there is an oxygen atom between each silicon bond
True or false: the properties of sand are very similar to the properties of a diamond
True!
True or false - sand cannot contain impurities
False - it can contain many impurities like calcium carbonate
What is the structure of graphite
Hexagonal rings bonded together by covalent bonds. Each Carbon atom is bonded to 3 others. The layers are attracted to each other by intermolecular forces
Why is graphite soft and slippery
Intermolecular forces between layers are weak
Layers easily move and slide over each other
Why can graphite conduct electricity
Each carbon atom only bonds to three others
There is a delocalised electron per carbon atom
These can carry heat and electricity
Graphite can conduct electricity
What is the name for one layer of graphite
Graphene
Uses of graphite
Pencils
Absorbs neutrons in nuclear reactions
Structure of fullerenes
Connecting hexagonal rings. However, some rings may have 5 or 7 carbon atoms. They can form a cage like/ball structure, or a tube