SL Chapter 10
0.0(0)
Card Sorting
1/136
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
1
New cards
What is a homologous series?
A homologous series is a set of organic compounds that have the same:
- general formula/functional group.
- similar chemical properties.
- The physical properties and the Mr will differ
- general formula/functional group.
- similar chemical properties.
- The physical properties and the Mr will differ
2
New cards
What are Hydrocarbons structurally?
These are non-polar, covalent compounds that are held together through weak Van-De-Waals forces.
Higher SA of interaction between the molecules \= stronger the forces \= higher BP for longer molecules.
Higher SA of interaction between the molecules \= stronger the forces \= higher BP for longer molecules.
3
New cards
Successive members in a homologous series differ by
CH2
4
New cards
What are the physical properties of all homologous series?
- Low melting points \= simple molecular covalent bonding as only the IM forces need to be broken.
- Small molecules (at the start) are gasses and then become liquids as the molecules become larger.
- Increasing boiling points as the molecule becomes bigger as there are more IM forces that need to be broken.
- Small molecules (at the start) are gasses and then become liquids as the molecules become larger.
- Increasing boiling points as the molecule becomes bigger as there are more IM forces that need to be broken.
5
New cards
Where do all organic compounds originate from?
- They all originate from crude oil ( a mixture of alkanes).
- This can be separated through fractional distillation through a fractional column.
- This can be separated through fractional distillation through a fractional column.
6
New cards
How does fractional distillation work?
1) Crude oil is heated within a furnace to vaporise most of the fractions.
2) These are then passed into a fractional column which has a temperature gradient. It is coolest at the top and hottest at the bottom.
3) Those with the lowest boiling points are collected at the top of the column whilst those with the highest boiling points are collected at the bottom.
4) They are collected at the different levels in the fractional column to produce the different homologous series.
2) These are then passed into a fractional column which has a temperature gradient. It is coolest at the top and hottest at the bottom.
3) Those with the lowest boiling points are collected at the top of the column whilst those with the highest boiling points are collected at the bottom.
4) They are collected at the different levels in the fractional column to produce the different homologous series.
7
New cards
What is empirical formula?
- This is the simplest whole number ratio of atoms that the compound will contain.
8
New cards
What is the molecular formula?
- The actual number of atoms that are present within a compound/molecule.
M \= (molar mass of empirical formula)n
where n is a scalar.
M \= (molar mass of empirical formula)n
where n is a scalar.
9
New cards
What is the structural formula?
- A representation of the molecule showing how the atoms are bonded to each other.
10
New cards
What are the types of structural formula?
Full structural: This shows all of the bonds between all of the atoms.
Condensed structural: Omits the bonds that can be assumed and contains the minimum amount of information without losing information.
Stereochemical: Shows the relative positions in 3 dimensions. (Large wedge \= sticking out from the page, dotted line \= behind the page, normal line \= in the line of the paper)
Skeletal \= Assumes the bonds between carbons exist and that there is H bonded to them as well.
Condensed structural: Omits the bonds that can be assumed and contains the minimum amount of information without losing information.
Stereochemical: Shows the relative positions in 3 dimensions. (Large wedge \= sticking out from the page, dotted line \= behind the page, normal line \= in the line of the paper)
Skeletal \= Assumes the bonds between carbons exist and that there is H bonded to them as well.
11
New cards
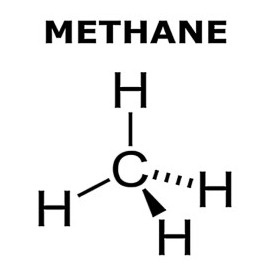
Stereochemical formula of methane
12
New cards
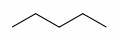
skeletal formula of pentane
13
New cards
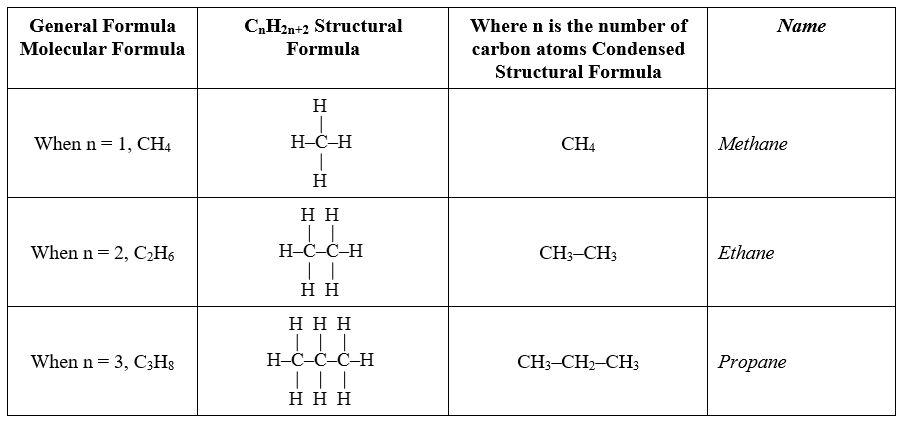
Condensed structural + full structural of the alkanes
14
New cards
What is the IUPAC system?
- This is the naming system for the longest chain for carbons in the compound. THESE CAN BE BENT MORE THAN 1 TIMES.
- This may also mean counting many different chains just to ensure you have the longest one.
- This may also mean counting many different chains just to ensure you have the longest one.
15
New cards
What are the names in the IUPAC system?
1 - Meth
2 - Eth
3 - Prop
4 - But
5 - Pent
6 - Hex
2 - Eth
3 - Prop
4 - But
5 - Pent
6 - Hex
16
New cards
What does R and R'/R1 and R2 mean?
- This means the connecting hydrocarbon chains can be either the same or different.
17
New cards
Alkane
Suffix \= -ane
General formula \= Cn H2n+2
E.g. Ethane \= C2H6
General formula \= Cn H2n+2
E.g. Ethane \= C2H6
18
New cards
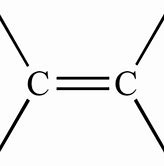
Alkene
Name of functional group \= alkenyl
Suffix \= -ene
General formula \= Cn H2n
e.g. Ethene
Suffix \= -ene
General formula \= Cn H2n
e.g. Ethene
19
New cards
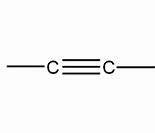
Alkyne
Name of functional group \= alkynyl
Suffix \= -yne
General formula \= Cn H2n-2
e.g. Ethyne
Suffix \= -yne
General formula \= Cn H2n-2
e.g. Ethyne
20
New cards
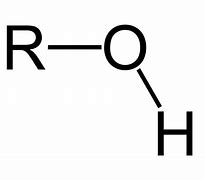
Alcohol
Name of functional group \= hydroxyl
Suffix \= -anol
General formula \= Cn H2n+1 OH
e.g. Ethanol
Suffix \= -anol
General formula \= Cn H2n+1 OH
e.g. Ethanol
21
New cards
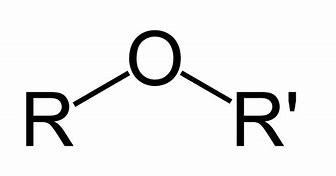
Ether
Name of functional group \= ether
Suffix \= -oxy- alkane
General formula \= R - O - R'
e.g. Methoxyethane
Suffix \= -oxy- alkane
General formula \= R - O - R'
e.g. Methoxyethane
22
New cards
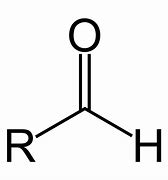
Aldehyde
Name of functional group \= Aldehyde
Suffix \= -anal
General formula \= R - CHO
e.g. Propanal
Remember as it has a H in the name as it does in the formula.
Suffix \= -anal
General formula \= R - CHO
e.g. Propanal
Remember as it has a H in the name as it does in the formula.
23
New cards
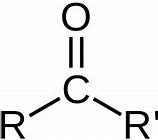
Ketone
Name of functional group \= Carbonyl
Suffix \= -anone
General formula \= R - CO - R'
e.g. Propanone
Suffix \= -anone
General formula \= R - CO - R'
e.g. Propanone
24
New cards
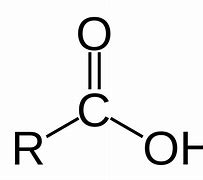
Carboxylic acid
Name of functional group \= Carboxyl
Suffix \= -anoic acid
General formula \= Cn H2n+1 COOH
e.g. Propanoic acid
Suffix \= -anoic acid
General formula \= Cn H2n+1 COOH
e.g. Propanoic acid
25
New cards
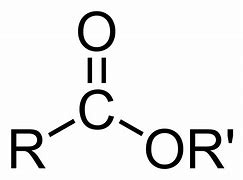
Ester
Name of functional group \= Ester
Suffix \= -anoate
General formula \= R - COO - R'
e.g. Methyl Propanoate
Suffix \= -anoate
General formula \= R - COO - R'
e.g. Methyl Propanoate
26
New cards
How are esters formed?
- When the functional group of an alcohol replaces the hydrogen of a carboxylic acid in a condensation reaction.
27
New cards
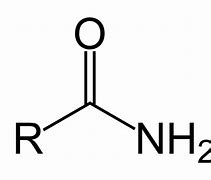
Amide
Name of functional group \= Carboxyamide
Suffix \= -anamide
e.g. Propanamide
Suffix \= -anamide
e.g. Propanamide
28
New cards
Nitrile
Name of functional group \= Nitrile
Suffix \= -anenitrile
e.g. Propanenitrile
Suffix \= -anenitrile
e.g. Propanenitrile
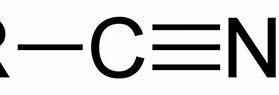
29
New cards
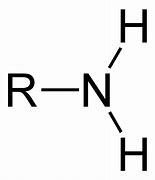
Amine
Name of functional group \= Amine
Suffix \= -anamine
e.g. Ethanamine
Suffix \= -anamine
e.g. Ethanamine
30
New cards
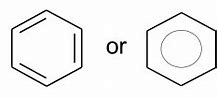
Arene
Name of functional group \= Phenyl
Suffix \= -benzene
e.g. Methylbenzene
Suffix \= -benzene
e.g. Methylbenzene
31
New cards
What are the different types of side chains?
- Alkane
- Halogenoalkane
- Amine
- Halogenoalkane
- Amine
32
New cards
Alkane side chains
Prefix - IUPAC + yl
33
New cards
Halogenoalkane side chains
Prefix - Fluoro, chloro, bromo, iodo...
Functional group - Halogeno
Functional group - Halogeno
34
New cards
Amine side chains
Prefix - amino
Functional group - Amine
Functional group - Amine
35
New cards
When adding side chains to the formula what are the steps?
What - what is the side chain
Where- Where is the side chain attached
Where- Where is the side chain attached
36
New cards
How to determine where the side chains are?
- Count the carbons. Always use the shortest way from the 2 and maintain that direction if there are multiple.
- If there are many add di, tri etc.
- Specify where they are before the name. If there are multiple use a comma to separate this.
- Oder them alphabetically by the names (e.g. ethyl will come before methyl in a compound).
- If there are many add di, tri etc.
- Specify where they are before the name. If there are multiple use a comma to separate this.
- Oder them alphabetically by the names (e.g. ethyl will come before methyl in a compound).
37
New cards
When do you need to specify where the functional group is?
- This is when it can be anywhere but at the end. This number should be between the IUPAC prefix and the suffix.
38
New cards
What is a structural isomer?
- These are chemicals that have the same molecular formula but have different structural formulas (arrangement of the atoms).
39
New cards
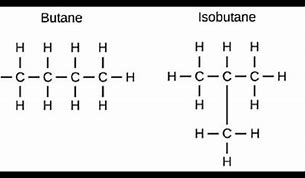
Example of a structural isomer?
C4H8 \= 2 different structural formulas.
- Butane
- 2-methylpropane
- Butane
- 2-methylpropane
40
New cards
Why are structural isomers important to the petrol industry?
- The distinction between branched and and straight chain alkanes has become vital.
- The more branched chains an isomer has, the more smoothly it will burn compared to straight chain isomers.
- These are then considered more premium with a HIGHER OCTANE NUMBER.
- The more branched chains an isomer has, the more smoothly it will burn compared to straight chain isomers.
- These are then considered more premium with a HIGHER OCTANE NUMBER.
41
New cards
Different types of structural Isomers
Chain isomers \= When the chain is arranged differently (branched)
Position Isomers \= When the functional group is in a different position
Functional Isomers \= When the functional group is different (Ketones vs aldehydes)
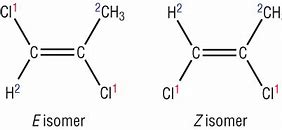
E-Z isomers \= Only in alkenes, when the atoms with the higher atomic number are on the same side it is the Z. When they are on the opposite side they are the E form.
This only occurs due to the limited rotation with the double bond.
Position Isomers \= When the functional group is in a different position
Functional Isomers \= When the functional group is different (Ketones vs aldehydes)
E-Z isomers \= Only in alkenes, when the atoms with the higher atomic number are on the same side it is the Z. When they are on the opposite side they are the E form.
This only occurs due to the limited rotation with the double bond.
42
New cards
If an isomer is more branched then...
- It will have a lower boiling point.
- The SA decreases and so do the Van De Waal forces allowing for them to be overcome easily.
- The SA decreases and so do the Van De Waal forces allowing for them to be overcome easily.
43
New cards
What are alkanes?
These are saturated, non-polar compounds that are mostly unreactive due to their sigma bonds.
44
New cards
Complete and incomplete combustion of alkanes
Complete \= Exothermic with CO2 and H2O released due to excess O2.
Incomplete \= Limited O2 leading to the formation of C and CO.
Incomplete \= Limited O2 leading to the formation of C and CO.
45
New cards
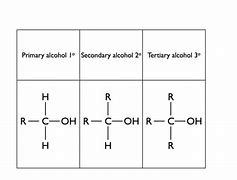
What are the different types of compounds?
Primary - The functional group is bonded to a carbon that is bonded to 1 other carbon and at least 2 H.
Secondary - The functional group is bonded to a carbon that is bonded to 2 other carbons and at least 1 H.
Tertiary - The functional group is bonded to a carbon that is bonded to 3 other carbons with no H.
Secondary - The functional group is bonded to a carbon that is bonded to 2 other carbons and at least 1 H.
Tertiary - The functional group is bonded to a carbon that is bonded to 3 other carbons with no H.
46
New cards
What is the only compound this classifying doesn't apply to?
Compounds that have only 1 carbon such as methanol.
47
New cards
How to draw skeletal formula?
- Count the number of carbons drawn as you draw it.
- For seperate functional groups draw another line with it at the end.
- For double/triple bonds add extra lines.
- For seperate functional groups draw another line with it at the end.
- For double/triple bonds add extra lines.
48
New cards
What does tetra mean?
four
49
New cards
Fermentation
- Sugars are converted into ethanol.
- A sugar solution, and yeast is left in a warm place.
- The CO2 gas escapes as gas and the ethanol can be distilled off due to its lower BP.
C6H12O6 --\> 2C2H5OH + 2 CO2
- A sugar solution, and yeast is left in a warm place.
- The CO2 gas escapes as gas and the ethanol can be distilled off due to its lower BP.
C6H12O6 --\> 2C2H5OH + 2 CO2
50
New cards
What are the 2 types of biofuels?
Bioethanol - from sugars
Biodiesel - from fats
Biodiesel - from fats
51
New cards
Advantages and disadvantages of biofuels
Advantages:
- They are renewable: new plants grown + use of animal waste.
- They are carbon neutral: Co2 released reabsorbed by plants grown.
Disadvantages:
- Land use: destruction of environments and taking space for food.
- Use of resources: Large use of water and fertilizer (which can pollute local water)
- They are renewable: new plants grown + use of animal waste.
- They are carbon neutral: Co2 released reabsorbed by plants grown.
Disadvantages:
- Land use: destruction of environments and taking space for food.
- Use of resources: Large use of water and fertilizer (which can pollute local water)
52
New cards
What are arenes?
- These are a branch of organic chemistry called aromatics.
- These are derived from/contain a benzene (C6H6)
- These are derived from/contain a benzene (C6H6)
53
New cards
What are aliphatics?
- These are straight chain organic compounds ( basically all compounds that don't have a ring/cyclical structure.
- These have different characteristics to aromatics.
- These have different characteristics to aromatics.
54
New cards
What is the key difference with benzene?
- It has no isomers.
- It doesn't undergo any addition reactions only substitution reactions.
- It doesn't undergo any addition reactions only substitution reactions.
55
New cards
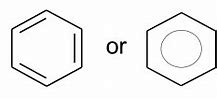
What are the 2 ways to draw the benzene structure?
- The kekule structure
- The true structure
- The true structure
56
New cards
How to name aromatic compounds?
- If a side chain is added on and not a functional group THEN 'side chain name' + benzene
- If a functional group is added to benzene THEN it is a phenyl group \= phenyl + functional group suffix.
- If a functional group is added to benzene THEN it is a phenyl group \= phenyl + functional group suffix.
57
New cards
What is 1 exception to the bonding rule?
- Carboxylic acids \= They have the benzene structure with a methanoic acid coming off of this.
- SO it is called bezanoic acid.
- SO it is called bezanoic acid.
58
New cards
What structure should be drawn?
- NEVER the Kekule structure unless asked to.
59
New cards
What does the kekule structure suggest about the shape?
- It has 3 single c-c bonds and 3 double c-c bonds with hydrogen attached to each one.
60
New cards
Why is the kekule structure wrong?
- Bond length: Kekule \= single bonds longer and double bonds are shorter.
Real \= All the same lengths
- Hydrogenation: Kekule \= exothermic as the double bond will be broken to allow the 6H to be added.
Real: More energy is needed making it endothermic as benzene is more stable.
- Addition/substitution reactions: Kekule \= can do both.
Real \= Only substitution.
- Shape: Kekule \= not symmetrical
Real \= Perfectly symmetrical creating a perfect hexagon.
Real \= All the same lengths
- Hydrogenation: Kekule \= exothermic as the double bond will be broken to allow the 6H to be added.
Real: More energy is needed making it endothermic as benzene is more stable.
- Addition/substitution reactions: Kekule \= can do both.
Real \= Only substitution.
- Shape: Kekule \= not symmetrical
Real \= Perfectly symmetrical creating a perfect hexagon.
61
New cards
Summary of why kekule was wrong
- The bonds are all the same length
- Benzene is more stable than he thought
- Can only undergo substitution
- Is perfectly symmetrical is shape.
- Benzene is more stable than he thought
- Can only undergo substitution
- Is perfectly symmetrical is shape.
62
New cards
What does the circle in the middle of the structural formula represent?
- The delocalised pi bonds/the cloud of 12 e^- above and below the benzene ring due to pi bonds.
63
New cards
What is the structure of benzene?
- It is cyclic
- Bond angles are at 120 degrees making it planar (flat).
- Bond angles are at 120 degrees making it planar (flat).
64
New cards
Bonding/structure of benzene
- Each C atom has sp^2 hybridised to form 3 sigma bonds (with 2 C and H)
- 1 unhybridized p electron on each carbon atom which overlap and are shared over the 6 carbon atoms evenly.
- This creates a delocalised pi electron cloud above and below the atoms.
- 1 unhybridized p electron on each carbon atom which overlap and are shared over the 6 carbon atoms evenly.
- This creates a delocalised pi electron cloud above and below the atoms.
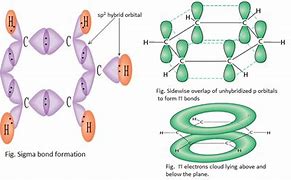
65
New cards
Why is benzene so stable?
This is due to the 'resonance energy' coming from the delocalised e^- in the PI clouds above and below the ring.
66
New cards
Explain sp2 hybridization
- This resonant hybrid has an sp2 hybridisation where the s and the p orbitals merge.
- This means that an e^- from the s orbital jumps to fill the empty space in the p orbital. This leaves the 2s orbital empty, and a hybrid orbital between the s and p orbital that has 3 unpaired e^-.
- 1 e^- is left in the p orbital and that is the 1 in the pi cloud.
- This means that an e^- from the s orbital jumps to fill the empty space in the p orbital. This leaves the 2s orbital empty, and a hybrid orbital between the s and p orbital that has 3 unpaired e^-.
- 1 e^- is left in the p orbital and that is the 1 in the pi cloud.
67
New cards
What are sigma bonds?
- These are side on s orbitals that create single bonds.
68
New cards
What are pi bonds?
- These are parallel p orbitals.
- With sigma bonds create double bonds.
- With sigma bonds create double bonds.
69
New cards
When drawing cyclic structures the side chains...
- Don't matter where they go as long as there relative position near each other matches
70
New cards
When writing the structural condensed formula...
- Include side chains in brackets to show that they are side chains.
71
New cards
How does the Hydrocarbon skeleton affect the boiling point of organic molecules?
- The longer the chain of hydrocarbons is \= more London Dispersion forces (IM) \= high boiling point
- The more branched the hydrocarbon \= less contact with the other hydrocarbons \= less/weaker London Dispersion forces (IM)
- The more branched the hydrocarbon \= less contact with the other hydrocarbons \= less/weaker London Dispersion forces (IM)
72
New cards
How does the functional group effect the boiling point of organic molecules?
- The functional group can form H bonds or dipole-dipole (polar covalent bonds). or London dispersion forces.
- These have highest BP with H bonds and lowers BP with London Forces.
- These have highest BP with H bonds and lowers BP with London Forces.
73
New cards
What is the order of the functional groups i using lowest to highest BP?
- Branched Alkane (London forces)
- Alkane (London Forces)
- HalogenoAlkane (Dipole-Dipole forces)
- Aldehyde (Dipole-Dipole forces)
- Ketone (Dipole-Dipole forces)
- Alcohol (Hydrogen bonds)
- Carboxylic acid (Dipole-Dipole forces + Hydrogen bonds)
- Alkane (London Forces)
- HalogenoAlkane (Dipole-Dipole forces)
- Aldehyde (Dipole-Dipole forces)
- Ketone (Dipole-Dipole forces)
- Alcohol (Hydrogen bonds)
- Carboxylic acid (Dipole-Dipole forces + Hydrogen bonds)
74
New cards
What are the main 3 elements that will mean a dipole-dipole force in organic molecules?
- F (fluorine)
- O (oxygen)
- Cl (chlorine)
- O (oxygen)
- Cl (chlorine)
75
New cards
What are the positives of Alkanes?
- These can be used as a base chemical when derived from crude oil to make other organic compounds.
- It can act as fuel for heating + transport + electricity production.
- It can act as fuel for heating + transport + electricity production.
76
New cards
What are the negatives of Alkanes?
- They release CO2 and CO into the atmosphere that can cause climate change as well as respiratory issues (Health issues)
- When used as fuels in cars often mean incomplete combustion releasing small C molecules into the the atmosphere causing global dimming.
(When the UV light from the sun is blocked)
- When used as fuels in cars often mean incomplete combustion releasing small C molecules into the the atmosphere causing global dimming.
(When the UV light from the sun is blocked)
77
New cards
What are the ONLY reactions that the Alkanes can go through?
- Substitution reactions where 1 atom/molecule is take off of the compound and is replaced with something else.
- A hydrogen atom can be replaced with a halogen atom to create a halogenoalkane.
- A hydrogen atom can be replaced with a halogen atom to create a halogenoalkane.
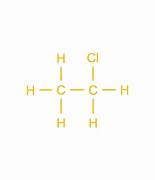
78
New cards
what type of reaction is this?
nucleophilic substitution
79
New cards
What is needed to do this reaction?
- The C-H bond is very strong so it requires a halogen radical to start.
80
New cards
What is a radical?
- An atom or compound with an unpaired electron at a high energy, uncharged.
- Usually Cl is used to make halogenoalkanes.
- These are presented by their formula and a dot next to them to show the free e^-.
- Usually Cl is used to make halogenoalkanes.
- These are presented by their formula and a dot next to them to show the free e^-.
81
New cards
How are curly arrows used in an equation?
- These are used to show the movement of the e^- in reactions. Especially those that will be used to form a radical or those from a radical.
- 1/2 an arrow head represents the movement of 1 e^-. A whole arrow head represents the movement of 2 e^-.
- These show where the e^- are to where they will be.
- 1/2 an arrow head represents the movement of 1 e^-. A whole arrow head represents the movement of 2 e^-.
- These show where the e^- are to where they will be.
82
New cards
What are they 3 steps for halogenation?
- Initiation/photochemical homolytic fission: Radicals are made
- Propagation: The continuation of the growth of radicals.
- Termination: Where the radicals stop being produced.
- Propagation: The continuation of the growth of radicals.
- Termination: Where the radicals stop being produced.
83
New cards
Initiation/photochemical homolytic fission
- When the bond of a halogen (Cl2, Br2 etc.) breaks evenly to form 2 radicals with 1 unpaired electron each.
- This can only happen with halogens.
- UV light must be present as it has a high frequency/energy to break the strong covalent bond.
- Absorb a photon of UV light giving them high energy.
- This can only happen with halogens.
- UV light must be present as it has a high frequency/energy to break the strong covalent bond.
- Absorb a photon of UV light giving them high energy.
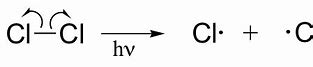
84
New cards
Propagation
- This is the process in which radicals are perpetually created making this a chain reaction ( it cannot be stopped and 1 reaction leads to another).
- One of the products will become 1 of the reactants.
e.g. Methane + Chlorine radical \= CH3 radical + HCL
CH3 radical + Cl2 \= Ch3Cl + Cl radical
Ch3Cl + Cl radical \= HCL + CH2Cl radical etc.
- One of the products will become 1 of the reactants.
e.g. Methane + Chlorine radical \= CH3 radical + HCL
CH3 radical + Cl2 \= Ch3Cl + Cl radical
Ch3Cl + Cl radical \= HCL + CH2Cl radical etc.
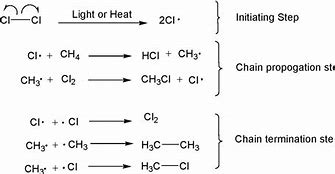
85
New cards
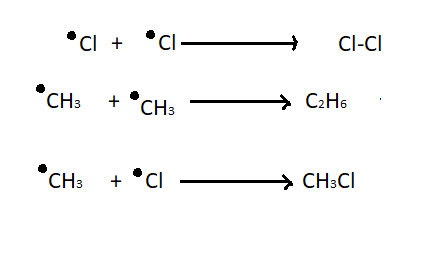
Termination
- This is when the chain reaction is stopped by 2 radicals incidentally reacting together to create no more radicals.
- BY CHANCE.
e.g. CH3 radical + Cl radical \= CH3Cl
- BY CHANCE.
e.g. CH3 radical + Cl radical \= CH3Cl
86
New cards
What does the halogen act as in this reaction?
- A catalyst as it is used and can come out of the process unchanged.
87
New cards
Why is this ineffective?
- It produces hundreds of different molecules that can be hard to individually seperate.
88
New cards
What is the difference between homolytic and heterolytic fission?
Homolytic fission \= identical products (e.g. 2 Cl radicals)]
Heterolytic \= different products (e.g. + and - Cl ion)
Heterolytic \= different products (e.g. + and - Cl ion)
89
New cards
What is an alkene?
- These are unsaturated hydrocarbons due to the c-c double bond.
- These double bonds are made of both Pi + Sigma bonds that are sp2 hybridized.
- These double bonds are made of both Pi + Sigma bonds that are sp2 hybridized.
90
New cards
What is the structure of an alkene?
- These have a triangular plana shape at 120 degrees.
- The Pi bond can be easily broken to create positions for 2 new bonds on the C atoms. \= addition reactions.
- The Pi bond can be easily broken to create positions for 2 new bonds on the C atoms. \= addition reactions.
91
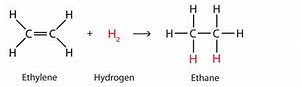
New cards
Hydrogenation of alkenes
- Reagents + conditions + product + example
- Reagents + conditions + product + example
Reagent: H2
Conditions: 150 degrees and a Ni catalyst present.
Product: An alkane
e.g. Ethene + Hydrogen \= Ethane
Conditions: 150 degrees and a Ni catalyst present.
Product: An alkane
e.g. Ethene + Hydrogen \= Ethane
92
New cards
Where is the hydrogenation of alkenes used?
- In the margarine industry to convert oils into saturated compounds with higher boiling points.
93
New cards
What is an electrophile?
- + ion or a molecule with a partially + charge (from a dipole) \= attracted to a negatively charged region + react by accepting a lone pair of e^- to form a covalent bond.
Phile \= loving - electron loving molecule that searches for e^-.
Phile \= loving - electron loving molecule that searches for e^-.
94
New cards
What is a nucleophile?
\- - ion or a molecule with a lone pair of e^- that can be donated to a + charged atom forming a covalent bond.
- Lone pair will override a + charge.
Nucleus loving \= searches out for nuclei.
- Lone pair will override a + charge.
Nucleus loving \= searches out for nuclei.
95
New cards
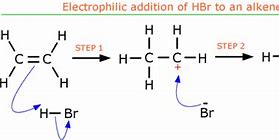
What is the mechanism for electrophilic addition of a pure halogen
1) Di-atomic Br gets closer to the double bond \= concentration of e^- repels the e^- in the Br bond causing an induced dipole.
2) The + Br causes the double bond to break \= Bond made with the slightly + Br ion.
This process forces an e^- to the + Br breaking that bond.
3) There is now a - Br ion and a carbo-cation. These then bond together.
4) This creates \-- dibromo-ane
2) The + Br causes the double bond to break \= Bond made with the slightly + Br ion.
This process forces an e^- to the + Br breaking that bond.
3) There is now a - Br ion and a carbo-cation. These then bond together.
4) This creates \-- dibromo-ane
96
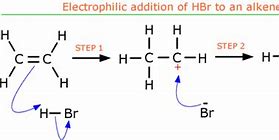
New cards
What to remember about electrophilic additions of pure halogens?
- These can happen with any alkene and any halogen.
- This is addition as 2 molecules react to form a larger molecule.
- This is addition as 2 molecules react to form a larger molecule.
97
New cards
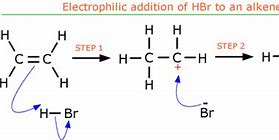
What is the mechanism for electrophilic addition of a hydrogen halide
1) H-Br gets closer to the double bond \= concentration of e^- repels the e^- in the bond causing an induced dipole.
2) The + H causes the double bond to break \= Bond made with the slightly + H ion.
This process forces an e^- to the + H breaking that bond.
3) There is now a - Br ion and a carbo-cation. These then bond together.
4) This creates bromoethane
2) The + H causes the double bond to break \= Bond made with the slightly + H ion.
This process forces an e^- to the + H breaking that bond.
3) There is now a - Br ion and a carbo-cation. These then bond together.
4) This creates bromoethane
98
New cards
Why do less reactive G7 become more reactive in this reaction?
- As the less reactive they are typically \= the easier it will be to break the bond and complete the reaction quicker.
99
New cards
Electrophilic addition of Bromine water
1) Br-Br gets closer to the double bond \= concentration of e^- repels the e^- in the bond causing an induced dipole.
2) The + Br causes the double bond to break \= Bond made with the slightly + Br ion.
This process forces an e^- to the + Br breaking that bond. The Br^- is left floating in the water.
3) A water molecule bonds to the carbocation. Although this means that the O has 3 bonds which it doesn't like.
4) One of the H from the bonded water molecule attached itself to another water molecule to make
H3O + Bromoalcohol.
Due to water being in a higher conc than the Br^- ions.
2) The + Br causes the double bond to break \= Bond made with the slightly + Br ion.
This process forces an e^- to the + Br breaking that bond. The Br^- is left floating in the water.
3) A water molecule bonds to the carbocation. Although this means that the O has 3 bonds which it doesn't like.
4) One of the H from the bonded water molecule attached itself to another water molecule to make
H3O + Bromoalcohol.
Due to water being in a higher conc than the Br^- ions.
100
New cards
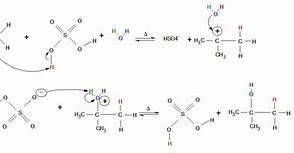
Electrophilic addition of water
1) H2SO4 gets closer to the double bond \= concentration of e^- repels the e^- in the bond causing an induced dipole.
2) The H from the H2SO4 is bonded to the ethene creating a carbocation and HSO4^- molecule.
3) Water joins onto the carbocation to make another molecule where the H2O is +.
HYDROLYSIS:
4) The HSO4^- ion takes the H from the bonded water molecule to make both of the molecules more stable.
5) This created H2SO4 and Ethanol.
2) The H from the H2SO4 is bonded to the ethene creating a carbocation and HSO4^- molecule.
3) Water joins onto the carbocation to make another molecule where the H2O is +.
HYDROLYSIS:
4) The HSO4^- ion takes the H from the bonded water molecule to make both of the molecules more stable.
5) This created H2SO4 and Ethanol.