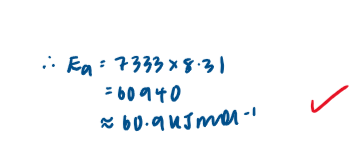Core Practical 14 : Determine the activation energy for the reaction between bromide ions and bromate(V) ions
0.0(0)
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 2:52 PM on 1/7/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
Overview:

2
New cards
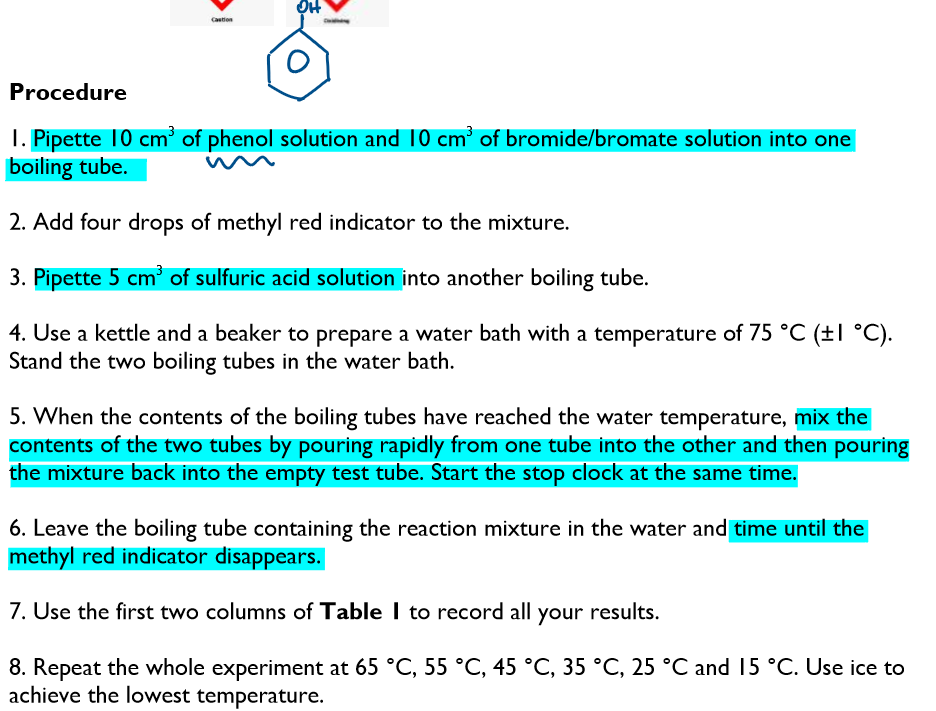
Procedure:
3
New cards
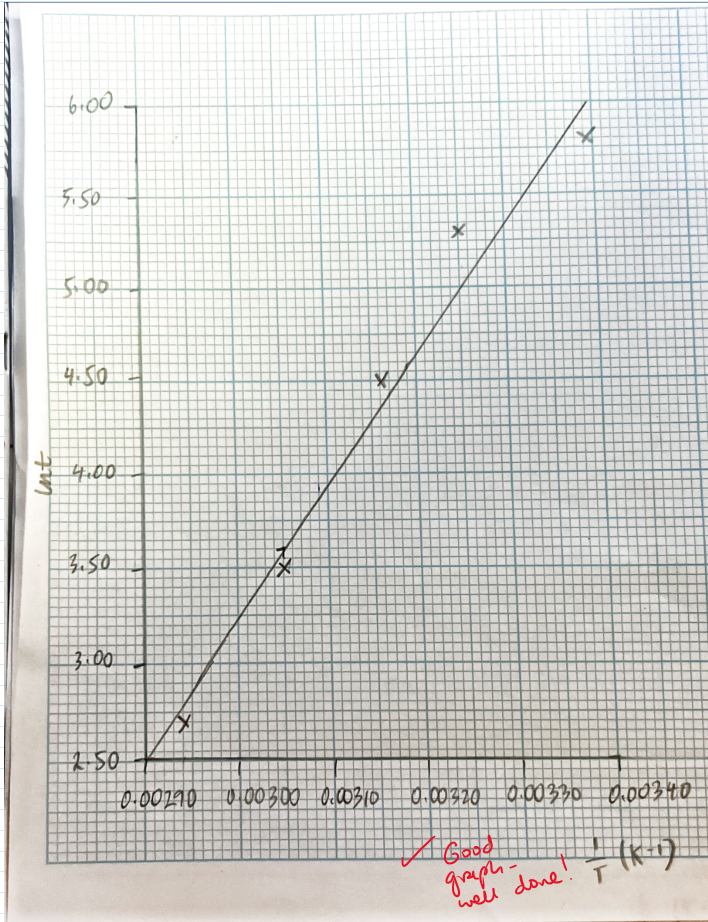
Plot a graph of ln t (y axis) against 1/T (x axis)
4
New cards
Learning tips:
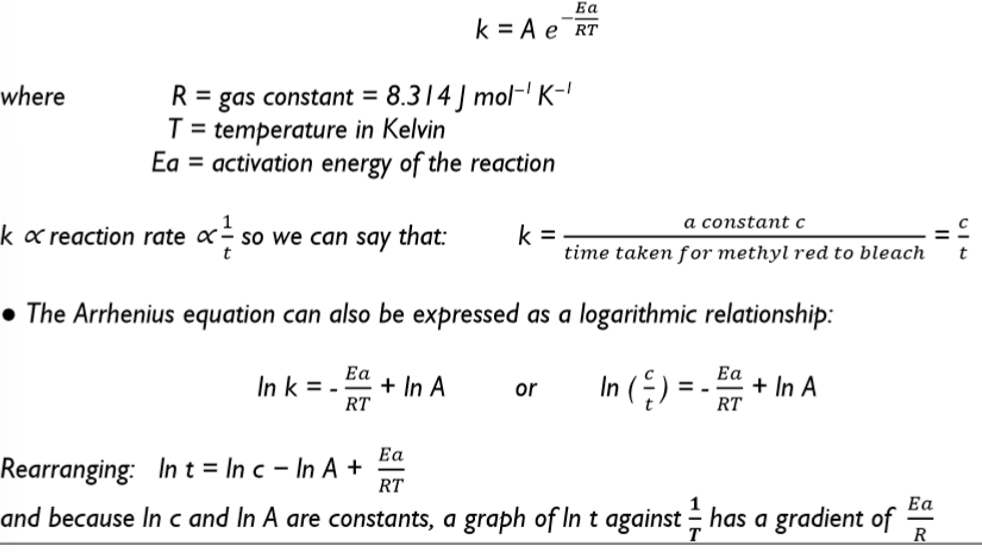
the Arrhenius equation is an exponential relationship between the rate constant, k, and temperature, T
5
New cards
Write an equation for the reaction between bromine and phenol.
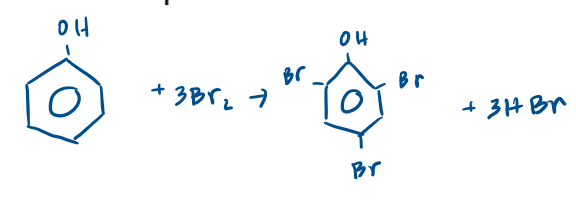
6
New cards
What function does the methyl red have in this experiment?
The methyl red serves to mark the end point of the reaction when it is bleached by the bromine, after the phenol is used up and Br2 remains in solution.
7
New cards
Measure the gradient of the graph:

8
New cards
Calculate the activation energy for this reaction, Ea