Lecture 13: Monoclonal Antibodies and Their Uses
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Antibodies
Made by plasma cells to recognise specific targets e.g. pathogen or infect cell - protect us against harmful infectious agents
Also useful as a detection agent
Very specific reagents - hypervariable regions
Large proteins - easily “tagged” and stick stuff on to:
identify where they bind to an antigen
what antigen they bind to!
How To Make Antibodies
Mimic what happens in an infection
Inject small samples of antigens (mg, usually protein, but no necessarily)
Immunised several times (over 10 weeks)
Booster shots are given to amplify the immune response and maximise the production of antibodies in the serum.
Helps maximise class switching, avidity, and affinity for the antibody.
The serum is enriched for specific antibodies against the injected antigen.
Polyclonal response: Serum still contains antibodies to other antigens.
Why are Antibodies Polyclonal
Antigens usually contain many different epitopes.
The immune response is polyclonal because each antigen has multiple epitopes that stimulate different B-cells.
Even though a more dominant antibody response may occur, antibodies targeting different epitopes will still be produced.
Therefore, the antibodies in the serum are polyclonal, meaning they target more than one antigen.
Anti-Toxins
Injecting animals with sub-lethal doses of toxins, which induced the production of antibodies in their serum.
Generated polyclonal antibodies to neutralise toxins.
The first antitoxin against diphtheria was developed in 1890 by Emile Adolf von Behring.
Diphtheria, a bacterial infection, was a major cause of death in children.
Von Behring's discovery earned him the 1901 Nobel Prize.
Anti-Venom
First successful antivenom was developed by Albert Calmette in 1895 using cobra venom.
He injected an animal with sub-lethal doses of venom and harvested its serum. This crude method is still used today.
Anti-venoms are still used for venomous bites/stings (snakes, spiders, scorpions, frogs, etc.).
Polyclonal Antibodies in Disease Outbreaks
Used to treat Ebola and COVID-19.
William Poole used anti-serum during the Ebola outbreak.
Serum from survivors was used to treat patients during the COVID-19 pandemic, providing passive immunity.
Convalescent Plasma
Blood plasma from people recovering from an infection (esp. if severe), may contain high levels of polyclonal antibodies.
It can confer passive immunity to recipients e.g. by neutralising viral particles.
Administered either as convalescent plasma (mixed) or as purified polyclonal antibodies (hyperimmune globulin)
Used during influenza pandemic of 1918, Ebola outbreak of 2014, and COVID-19 (2020/21 in the USA).
Hyperimmune globulin is still used for post-exposure prophylaxis against viral infections like hepatitis B, chickenpox, and rabies.
Monoclonal Antibodies
A breakthrough by Köhler and Milstein, providing antibody purity to one target.
They developed a protocol to immortalise clones of cells capable of making antibodies.
This is achieved by fusing a normal plasma B cell with a myeloma cell (a tumor cell) to generate a hybridoma.
The hybridoma contains the antibody-producing genes from the B cell and the immortal properties from the myeloma cell, allowing the production of vast quantities of the same antibody.
In Vivo Generation of Monoclonal Antibodies
Mice Immunisation: Mice are immunised with an antigen several times to enhance antibody class switching and increase affinity for the target.
Cell Extraction: Spleen cells are removed from the mouse because they contain antigen-specific B cells, which are mortal (finite lifespan) and therefore classified as primary cells.
Making Plasma Cells Immortal
Splenocytes (antigen-specific B cells, mortal) are fused in vitro with a myeloma cell line (immortal).
The myeloma line:
Has a purine enzyme deficiency → cannot metabolise purine
Cannot secrete immunoglobulin (Ig).
The fusion produces hybridomas:
Immortal from the myeloma cell.
Antibody-producing from the B cell.
Culture media is used to select for cells that can both survive and metabolise purines.
Only fused cells (hybridomas) are immortal, survive and make antibodies.
HAT Medium and Selection of Hybridomas
Cells cultured in HAT medium:
Contains Hypoxanthine, Aminopterin, and Thymidine.
Myeloma cells cannot survive in HAT (can't metabolise purines).
Splenocytes (B cells) are not immortal and die out.
Result:
Only fused hybridoma cells survive:
Can metabolise purine.
Are immortal.
Produce antibody.
This process is time-consuming and includes single-cell cloning into wells, ensuring that each well ideally contains one hybridoma → for clonality.
Some fusions will result in antibody production against the OG antigen and be the product on one B-cell
Screening Hybridomas for Monoclonal Antibodies
Each well ideally contains one hybridoma cell, → ensures the antibodies are monoclonal.
Screening is done to identify clones that produce antibodies against the original antigen/target.
Successful hybridomas:
Are immortal.
Produce antibodies of defined specificity.
These clones can be cultured to provide an unlimited supply of monoclonal antibodies.
Ideally generate immortal cell lines secreting monoclonal antibodies
Process can be conducted on a large industrial scale
Advantages of Monoclonal Antibodies
High specificity
Low cross-reactivity – no other targets
Standardised worldwide – Ab used in one lab will be the same as the Ab used in another lab across the globe
Unlimited supply
Used in immunoassays, diagnosis, tissue typing, separation of cell types, and clinical therapeutic agents
Issues Caused by Antibodies
Used in laboratory and the clinic, BUT in vivo can trigger immune responses - e.g. detected by Fc receptors or macrophages and act as an opsonin
NOT access the sites needed – Abs are large proteins – difficult in delivery
Causes off-target effects – often if there is a similar antigenic epitope located somewhere else in the body
Solution - use of bioengineering
Bioengineering Solutions for Antibody Production
Insect systems used to produce antibodies with distinct sugar structures, making them less likely to cross-react with human immunity.
Fab fragments (antigen-binding portions of antibodies) can be engineered for targeted function.
Specialised delivery systems are developed to help antibodies reach specific target cells effectively.
Plant-based systems:
Example: ZMapp, an Ebola treatment, was produced using nicotine plants.
Immunological Methods Using Monoclonal Antibodies
Affinity chromatography to purify molecules
Enzyme Linked ImmunoSorbant Assay (ELISA) and ELISpot
Haemagglutination
Immunodetection on tissue samples
Western Blotting
Flow cytometry
Magnetic cell separation
Affinity Chromatography
Often used to purify an antigen or protein, e.g. for preparing an inoculum/treatment
Antibody target bound to beads in a column
Addition of a mixture of molecules, e.g. culture medium serum, to allow for binding to occur - only the antigen of interest will bind to the antibody
Washing away will remove any unbound substance, and the beads will remain in the column
Addition of elution mixture to extract the purified compound
Way to purify and enrich for specific targets, e.g. used to purify and enrich convalescent plasma
Magnetic Separation of Cell Population: Immunoisolation
Often used in the lab to obtain purified cell populations.
May be used clinically, e.g. cellular immunotherapy
Usually, Abs are attached to little metal beads and apply a magnetic force to retain anything bound to the metal beads – antigens of interest will be retained in the magnetic force until the magnets are removed
Quick and easy process – may be used to purify DCs for a DC vaccine preparation - can target all the cells you don’t want – keep them attached to the magnet so that unbound cells will wash through
Can have positive or negative selection
Agglutination
Oldest method in immunology - Identify if antibody is present for an antigen
Visualise clumping- i.e. antigen/antibody complexes form
Commonly used in blood typing
Also to diagnose if a patient has HAD an infection
e.g. Typhoid fever- mix serum with a culture of Salmonella typhi - clumping indicates the patient either had or has an S. typhi infection.
(N.B. antibodies persist beyond the infection)
Haemagglutination
Blood type is dependent on the antigens on the RBC
Mixing reactions with different combinations of those antibodies to see whether or not clumping occurs, to then determine blood type
Visual process due to the presence of RBCs
Detecting Antibody-Antigen Interactions
Antibodies are colourless, but can be tagged:
Fluorescent markers, e.g. FITC
Metal ions
Enzyme markers that change colour when a substrate is added, e.g. peroxidase
ELISA
Often used clinically, e.g. viral diagnostics, levels of hormone, antibody, etc.
MOST COMMONLY USED DIAGNOSTICALLY AS OPPOSED TO OTHER METHODS
Used for serum, urine, supernatants etc., but can be used on cell lysates - often uses liquids
Direct ELISA
Simplest form
The sample to be tested is added to the well and sticks to the plastic.
Then add antibody, which is covalently linked to an antibody
The antibody will bind to any target in the plastic wells
Wash away anything unbound
Add substrate to see a colour change – can measure the absorbance of this
If a known standard is present can determine the absorbance for this standard curve and determine how much protein is present in the sample.
Increasing Sensitive to Antibody Detection
Signal amplification is achieved using a secondary antibody (an anti-antibody).
The secondary antibody is generated against and binds to the Fc region of the primary antibody.
Multiple secondary antibodies can bind to a single primary antibody, amplifying the detection signal.
Antibody Detection - Fluorescence: Direct vs Indirect
Direct fluorescence:
A single antibody is directly conjugated to a fluorescent molecule.
Indirect fluorescence:
A primary antibody binds to the antigen.
Then multiple fluorescently-labelled secondary antibodies bind to the primary antibody.
= Signal is amplified — useful for detecting low-abundance targets
Indirect ELISA
Well is coated with an antigen i.e. your sample
The specific antibody to be measured is added
Enzyme-conjugated 2°Ab is added
Substrate added and the amount of 2°Ab (absorbance) measured by ELISA reader
ELISpot (Enzyme Linked Immunospot)
highly sensitive assay used to measure the frequency of cytokine or antibody-secreting cells at the single-cell level.
Widely used during COVID to study the immune response and identify major B-cell targets.
Cells are cultured ± stimuli on a surface coated with a capture antibody.
After incubation, the cells are washed away, but their secreted antibodies/cytokines remain bound to the surface.
Detection is similar to ELISA, but instead of color change in solution, it uses a precipitating substrate to form visible spots.
Each spot corresponds to a single responding cell (e.g. cytokine-secreting or antibody-producing).
Results in wells with visible “blobs”—each representing an individual activated immune cell
Uses of ELISpot
Used to detect any secreted protein.
Commonly used to assess:
Frequency of specific T cell responses
Frequency of antibody-secreting cells during infection or in response to vaccination
Helps identify common epitopes that stimulate B-cell responses.
Useful in vaccine research, e.g. to study T cells producing IFN-γ, indicating an active immune response.
Immunohistochemistry and Immunofluroescence
Shows localisation of antigen, can be quantitative – shows the location of a responding cell
Fresh, frozen, fixed tissues; cultured cells etc.
Applications include:
DNA sequences on chromosomes.
Spatial-temporal patterns of gene expression within cells/tissues.
Not readily used diagnostically
Immunohistochemistry
Use of enzyme-substrate reaction to bind to specific targets
Pancreatic tissue – Islets of Langerhans -stained with anti-glucagon antibodies (on the left)(α cells) and anti-insulin antibodies on the right (βcells). Antibodies were conjugated with peroxidase enzyme and detected with substrate
Immunofluroescence
Counterstained with a fluorescent stain e.g. DAPI to highlight the nucleus – useful in allowing you to orientate yourself around the tissue and tells you if you have a positive cell
Western Blotting
Qualitative and quantitative analysis of protein expression
e.g. from tissue homogenates or cultured cells. E.g. to see if a cell expressed CD3
Can be used diagnostically
e.g. Identify if pathogen present e.g. Lyme disease, HIV
2 Principles Underlining Western Blotting
Electrophoresis
A protein mixture is dissociated and run through a gel under an electric current.
Smaller proteins travel further than larger ones.
Migration also depends on protein charge.
Results in a distinct banding pattern showing protein separation.
Antibody-Based Detection
Antibodies bind to specific target proteins on the gel.
This allows visualisation of the bands.
Detection methods include luminescence, counterstaining and other visualisation techniques.
Identifying Lymphocytes Using Flow Cytometry
Different types of lymphocytes may look morphologically simillar e but express different cell-surface proteins.
Antibodies can be used to detect these unique markers.
Flow cytometry is used to analyse these surface markers on cells.
For example:
T cells express CD3
B cells do not express CD3
This allows distinction between cell types using a relatively crude but effective method.
Flow Cytometry
Uses lasers to detect fluorescent antibodies bound to cells.
Antibody is added to a cell mixture, and different fluorochromes are added(fluorescent dyes).
Cells flow through a system one at a time, and a laser hits each individual cell, providing information on cell size and granularity, indicating its function
Light scattering:
Larger cells (e.g., mast cells) scatter light differently due to their size and granularity.
Smaller cells (e.g., lymphocytes) scatter light more efficiently and pass more light through.
Depending on the fluorochrome, cells may be excited with a laser with different photomultiplier tubes to detect the light emitted when the laser hits the fluorochrome
This generates scatter plots that help visualise cell populations based on size, granularity, and fluorescent marker expression.
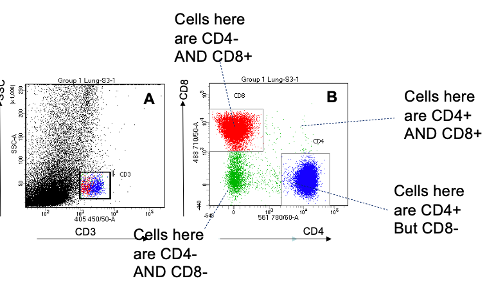
Scatter Plots
Generated from flow cytometry
Cells isolated from the lung were labelled with fluorescent antibodies against:
CD3 (T cells)
CD4 (T helper cells)
CD8 (Cytotoxic T cells)
then analysed by flow cytometry
Plot A: Granularity (side scatter) vs CD3
Helps identify general T cell populations.
Plot B: CD4 vs CD8
Allows a clear distinction between T helper and cytotoxic T cells.
The further along or up an axis = higher expression of that marker.
Uses of Flow Cytometry
Used often → generates quantitative and qualitative information e.g. different cell types in a blood or tissue sample
It is often used for
Levels of cell activation
Cell viability
Cytokines secreted
Rare cell types e.g. used to identify innate lymphoid cells
Clinical uses common
e.g. Diagnosis/staging of patients with a haematological neoplasm
detecting rare tumour cells
monitoring cell numbers (e.g. CD4 cells in HIV)
Monoclonal Antibodies At Home - Pregnancy Tests
Use ELISA-based technology to detect human chorionic gonadotropin (hCG), a hormone released from the placenta shortly after conception.
The original concept was created and patented by Margaret Crane in 1967.
Early pregnancy tests were crude, using hemagglutination reactions that looked for clumping of hCG in test tubes with mirrors to visualise the result.
Modern tests are more specific, using antibodies and an indicator reaction based on ELISA principles.
Monoclonal Antibodies at Home - COVID-19 Tests
Lateral flow tests use the same principles as pregnancy tests and ELISA
They employ monoclonal antibodies and lateral flow immunoassay technology, derived from ELISA concepts.
These tests are designed to detect viral proteins (antigens) using antibody-based capture and detection systems, providing a visible signal (e.g. a colored line) when the antigen is present.
Monoclonal Antibodies in the Clinic – TNF Therapy
Marc Feldmann and Ravinder Maini created TNF therapy, using monoclonal antibodies therapeutically.
Finola and her team hypothesised that active autoimmune diseases generate damaging cytokines, and that blocking one of these key cytokine may block all of the cytokines and the pro-inflammatory consequences
A control test using antibodies against lymphotoxin (LT) had no effect on IL-1 levels.
However, antibodies against TNF successfully blocked cytokine production, reducing inflammation and turning off part of the inflammatory cascade.
This approach was game-changing and highly successful in the clinic.
Uses TNF-a Therapy
Monoclonal antibodies to TNF-α now used for:
rheumatoid arthritis,
IBD,
Ankylosing spondylitis,
Psoriasis,
Psoriatic arthritis
Blockade of Immune Checkpoints to Enhance T-Cell Responses
James Allison and Tasuku Honjo were awarded the Nobel Prize for developing checkpoint inhibitor therapy, a form of antibody therapy used in cancer treatment.
Their research focused on understanding the immune response "off switches", particularly PD-1 and CTLA-4 (immune checkpoint molecules), which tumours exploit to suppress immune responses.
They generated antibodies to block PD-1 and CTLA-4, preventing the immune system from being switched off.
This allowed T-cells to remain ‘on’, boosting the immune response to help kill tumour cells.
Method of boosting the immune response to tumours
Checkpoint blockade is now a successful and a used therapeutic approach in oncology.