Things to know for Rates of reaction
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Rate of reaction
speed at which a reaction is taking place
3 requirements for collision theory
Particles must collide
Particles must collide with sufficient energy
Particles must collide with the correct orientation
What are the three musts in concentration graphs
Scale of graph
Ensuring stoichometric ratios are same
Ensuring they reach equilibrium at the same time
Temperature
Average translational kinetic energy of the system
Inc of Temperatures affect on rate of reaction
Temperature is the average translational kinetic energy,
Increasing temp hence increases kinetic energy
This increases speed, resulting in more collisions, collision more energetically
More particles above Ea
Which increases the chance of successful collisions, hence increasing rate of reaction.
Chemical energy
The bond energy in a substance
Increase in concentration
Increase in concentrations
Decreases the space
Proportion same
Increases frequency of collisions
Increases number of successful collisions
This increases rate of reaction
What does smaller particles do
Smaller particles greater surface area
what do you say instead of no of collisions
FREQUENCY
Inc in surface area

Inc in gas pressures

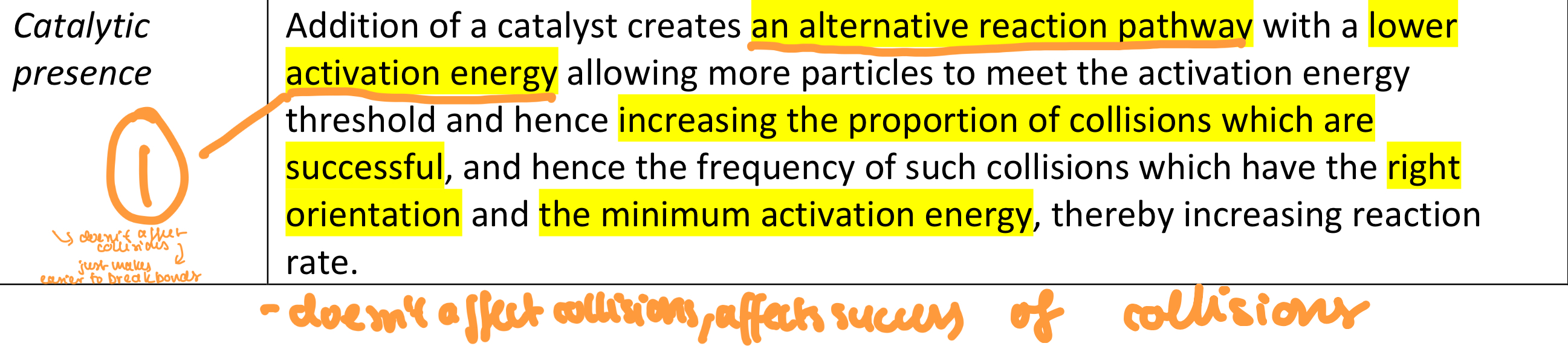
Catalysis prescience
What does pressure affect
the frequency of collisions
What does temperature affect?
Frequency of collisions, and proportion of successful collisions
What does surface area affect
The frequency of collisions
What does catalytic presence affect
The proportion of successful collisions
What does concentration affect?
The frequency of collisions
What does humidity affect in relation to rusting and corrosion of steel bars
Increased reactivity; more moisture.warer facilititates transfer of ions and electrons in corrosion process.
Conductivity; moisture can increase conductivity, and makes it easier for corrosion to occur
Oxygen availability; increased avalolability of oxygen in a humid environment as moisture can absorb more water, may prompt oxidation of Fe/iron in steel.
If volume increases but concentration stays the same what is the effect on the rate of reaction
There is no difference, because collisions are the same per unit area.
How could you determine rate in diff experiments
Mass lost (g), Scale
With syringe if gas
Change in pH