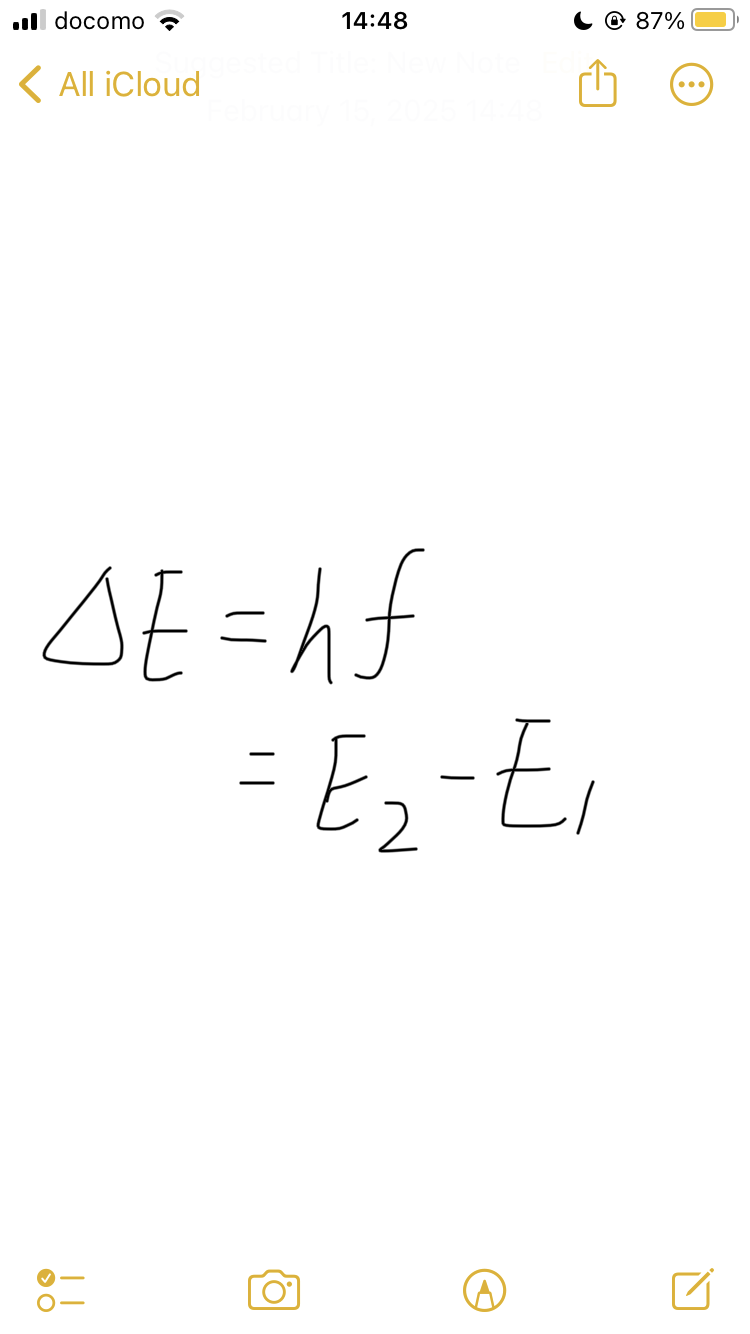A2 Physics Quantum Physics
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
what is a photon
a quantum of energy when the energy is in the form of electromagnetic radiation
formula for photon energy
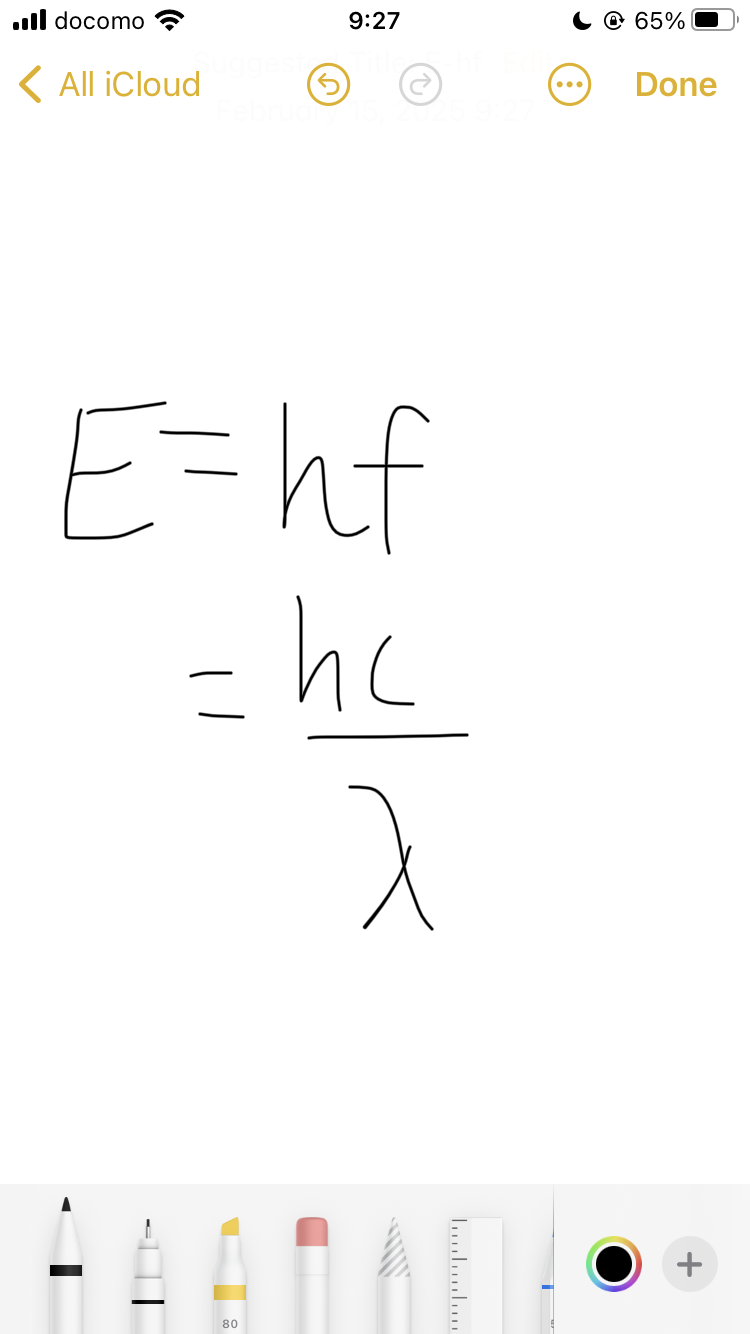
convert from electron volt (eV) to joules (J)
multiply by 1.6 × 10^-19
formula for momentum of photon
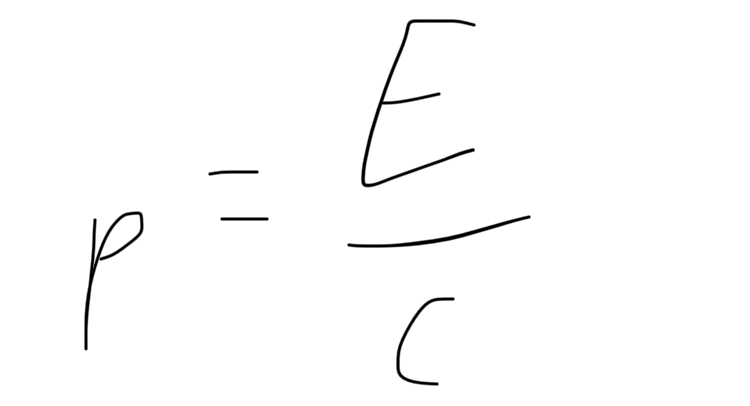
what are emitted from a metal surface when illuminated by electromagnetic radiation
photoelectrons
what is threshold frequency
minimum frequency of incident electromagnetic radiation required to remove a photoelectron
what is threshold wavelength
longest wavelength needed to remove a photoelectron
formula for photoelectric emission
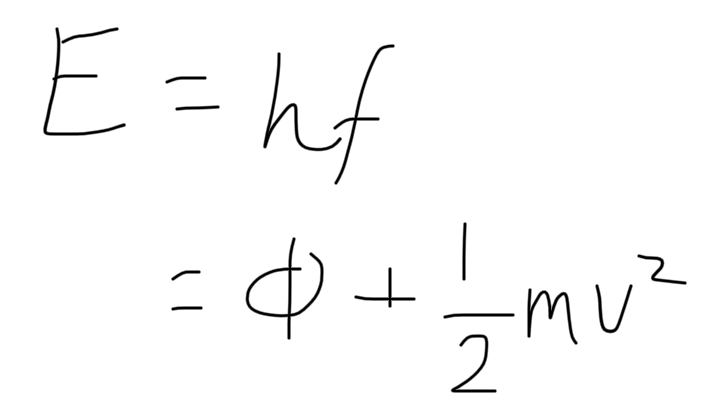
what is the effect of intensity on maximum kinetic energy of photoelectrons
there is no effect because increase in intensity only increases the number of photoelectrons leaving the surface
what is the effect of intensity on photoelectric current
they are proportional because photoelectric current refers to number of photoelectrons emitted which increases with intensity
what is photoelectric effect
emission of electrons when electromagnetic radiation is incident
what is work function energy
minimum energy for an electron to leave the surface
explain the evidence provided by the electron diffraction for the wave nature of particles
what is de Broglie wavelength
the wavelength associated with a moving particle
formula for de Broglie wavelength
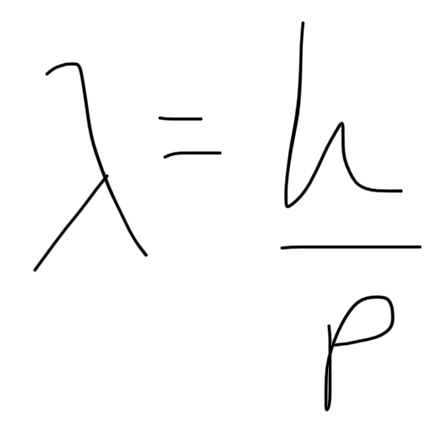
how do you describe the electron energy levels in isolated atoms
they are discrete
how does emission and absorption line spectra look like
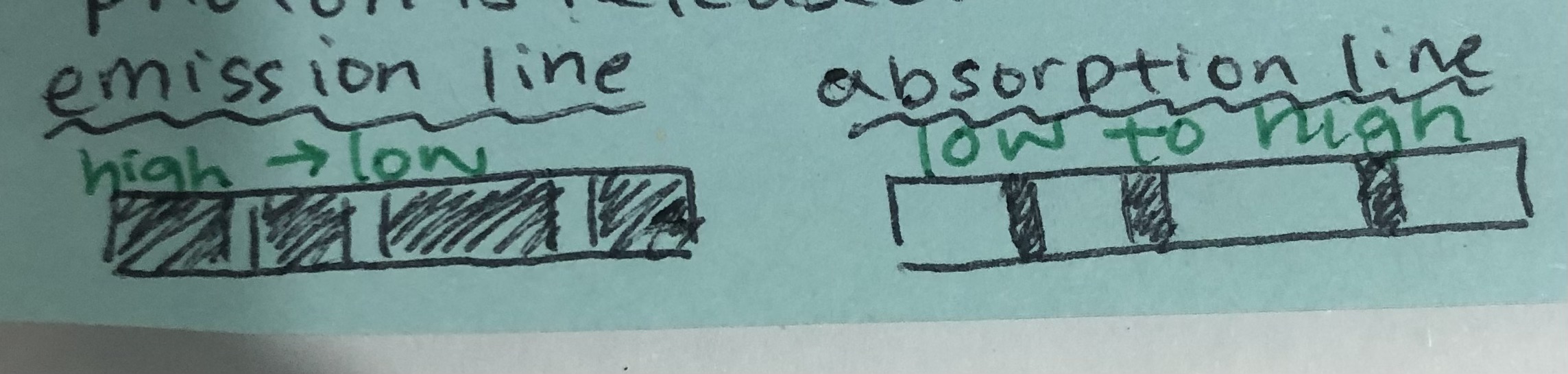
how is an emission spectra made
electron transitions from higher energy level to lower energy level → emission of photon
represented by coloured lines in a black background
how does an absorption spectra form
when an electron transitions from low to high energy → due to absorption of photon
a continuous spectrum with black lines at certain wavelengths
formula for difference between two energy levels