Topic 6: Organic chemistry I
1/58
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
what is a hydrocarbon
A compound which contains only hydrogen and carbon
what is a homologous series
homologous- follow the same general formula and have similar chemical properties
structural isommerism
compounds with the same molecular formula but different structural arrangement of the carbon chain
chain isomerism
compounds with the same molecular formula but their longest hydrocarbon chains are different
Positional isomerism
compounds with the same molecular formula but different positions of a functional group
e.g. Butan-1-ol and Butan-2-ol
chain isomerism
compounds with the same molecular formula but their longest hydrocarbon chains are different
Positional isomerism
compounds with the same molecular formula but different positions of a functional group
e.g. Butan-1-ol and Butan-2-ol
functional group isomerism
when different functional groups result in compounds having the same molecular formula
reforming and cracking of crude oil
processing straight chain alkanes into branched/cyclic alkanes for efficient combustion
e.g. pentane —> cyclopentane + H2
The breaking down of an unsaturated hydrocarbon into smaller hydrocarbons
e.g. octane —> pentane + propane
pollutants of incomplete combustion of alkane fuels
carbon monoxide,soot,
If hydrocarbon is impure: sulfur dioxide,nitrogen dioxide
cis/trans and e/z isomerism
Both forms of stereoisomerism
Cis/trans notation is for less complex compounds, If the 2 highest priority substituent are on the same side=cis If the 2 highest priority substituents are in opposite sides= trans
e/z notation is for more complex compounds based on priority
high priority refers to a higher atomic number, low priority refer to a low atomic number
If the high priority molecules are on the same side it’s Z notation(Z-same side) If it’s high priority on opposite sides it’s E notation
Alkane general formula
CnH2n+2
use of alternative fuels
Biodiesel and alcohols both derived from plants can be used a renewable fuel alternative
advantages
form of a renewable energy source as most other fuel source are finite/non renewable
no risk of large scale pollution
Disadvantages
less food crops may be grown as they need to be used for fuel instead
shortage of fertile soil as fuel is in high demand
land not used to grow food crops
reforming and cracking of crude oil
processing straight chain alkanes into branched/cyclic alkanes for efficient combustion
e.g. pentane —> cyclopentane + H2
The breaking down of an unsaturated hydrocarbon into smaller hydrocarbons
e.g. octane —> pentane + propane
what are the steps is free radical substitution
Initiation,propagation and termination
pollutants of incomplete combustion of alkane fuels
carbon monoxide,soot,
If hydrocarbon is impure: sulfur dioxide,nitrogen dioxide
free radical substitution mechanism

combustion of alkanes
alkane + oxygen = carbon dioxide + water
what problems can arise from the products of incomplete combustion
carbon monoxide can lead to poisoning if inhaled
sulfur dioxide can react with water vapour to produce acid rain (H2SO3)
nitrogen dioxide and soot can cause respiratory issues, and potentially acid rain
saturated vs unsaturated compunds
alkanes and cycloalkanes are saturated (no c=c bond)
alkenes and cycloalkenes are unsaturated (c=c bond)
use of catalytic converter
A catalytic converter reduces the atmospheric pollutants entering the air from the combustion of fuels, through the use of a catalyst e.g platinum
e.g. the oxidation of carbon monoxide into carbon dioxide
by doing reactions like this it reduces the the emission of harmful pollutants and can improve air quality
What is an electrophile
Electrophiles are electron deficient species and can accept an electron pair from electron rich species. Attracted to negative charge
use of alternative fuels
Biodiesel and alcohols both derived from plants can be used a renewable fuel alternative
advantages
form of a renewable energy source as most other fuel source are finite/non renewable
no risk of large scale pollution
Disadvantages
less food crops may be grown as they need to be used for fuel instead
shortage of fertile soil as fuel is in high demand
land not used to grow food crops
Reaction mechanism of alkenes with halogens
Alkenes react with halogens to form dihalogenoalkanes
Type of reaction: electrophilic addition
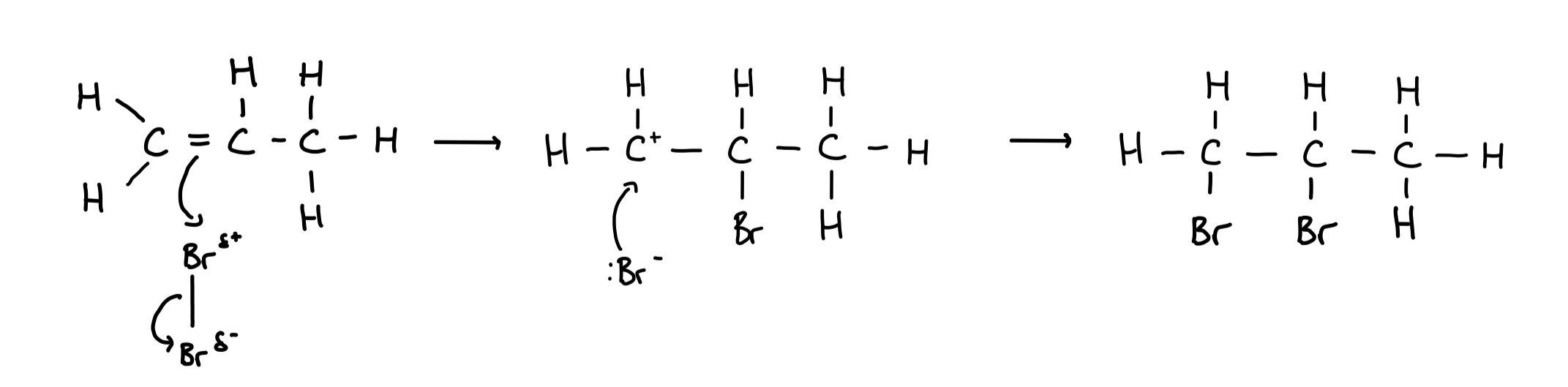
Reaction of alkenes with halides
Alkenes react with halogens to produce halogenoalkanes
Type of reaction: electrophilic addition
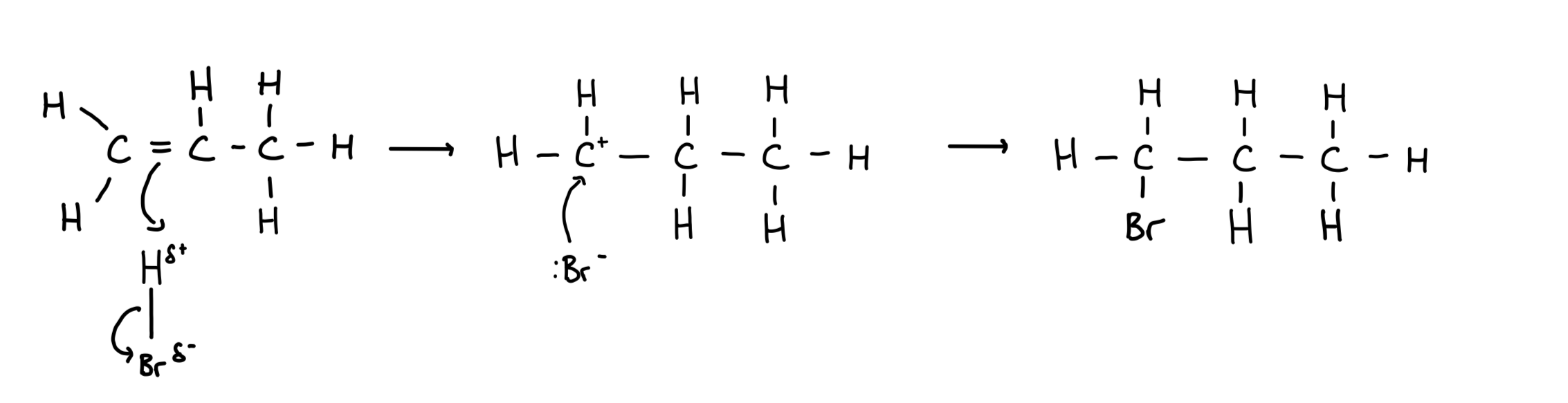
what is a radical and how is it formed
A radical is a species with an unpaired electron
It is formed by the homolytic fission of a covalent bond
Reaction of alkenes with potassium manganate (VII)
Alkenes react with potassium manganate (VII) in acidic conditions to produce a diol
Type of reaction: Oxidation
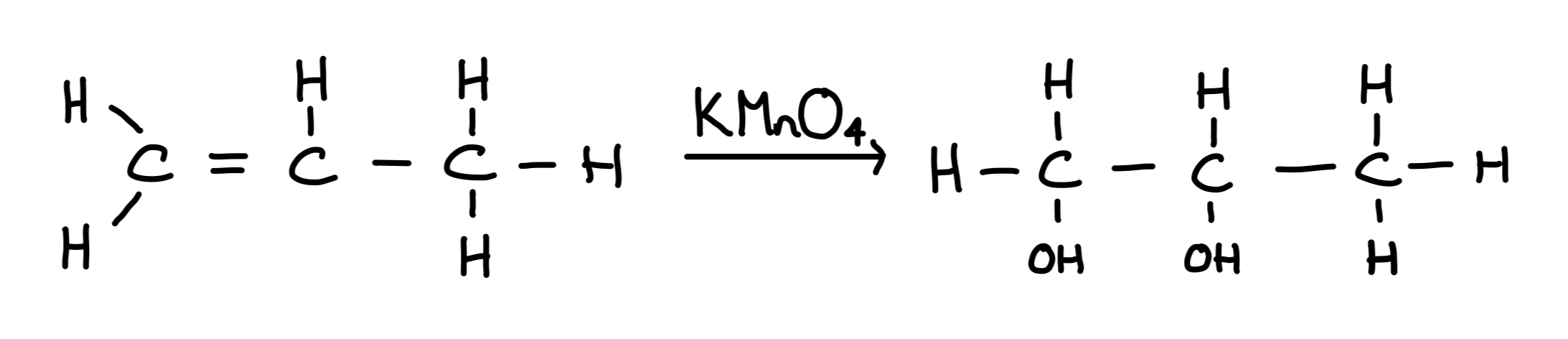
Carbocations stability
Tertiary>Primary>Secondary
Carbocation stability determines which products will me be made more frequently (major product), tertiary carbocations are the most stable and are often the major product
what are the steps is free radical substitution
Initiation,propagation and termination
limitations of free radical substitution
can lead to the formation of multiple products,including unwanted ones
radicals are very reactive and may react with unintended impurities
How do chemists limit the problem caused by polymer disposal
Creating biodegradable polymers
Biodegradable polymers produce less waste in the environment
They can be made from renewable resources,and have a reduced environmental impact
Reduces toxic gas from incineration of plastics
More advanced incineration techniques minimise toxic gas emissions during combustion
Catalytic converters also reduce harmful byproducts produced during combustion
What is a nucleophile
A nucleophile is a chemical species that donates an electron pair to form a covalent bond with an electron-deficient species . A species attracted to positive charge
combustion of alkanes
alkane + oxygen = carbon dioxide + water
bonding in alkenes
Bonding in alkenes consists of sigma and pi bonds. The pi bonds are formed by sideways overlap of p orbitals, creating an electron cloud (high electron density) above and below the plane of the carbon chain. Sigma bonds between the carbon atoms are formed by end-on overlap of sp2 orbitals.
C=C double covalent bond consists of one sigma (σ) bond and one pi (π) bond, the pi bonds are exposed therefore they have a high electron density making them more susceptible to attack from electrophiles.
Reaction of halogenoalkanes with ethanolic potassium hydroxide
Type of mechanism: elimination
reagent:potassium (or sodium) hydroxide
conditions:Heated,in ethanol
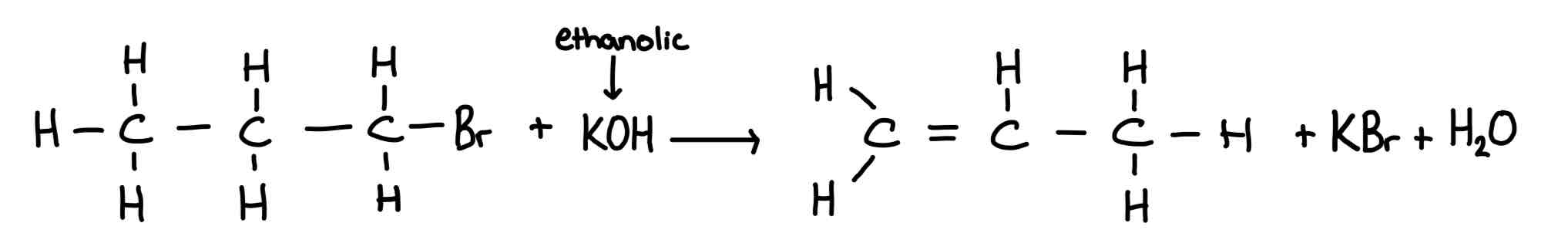
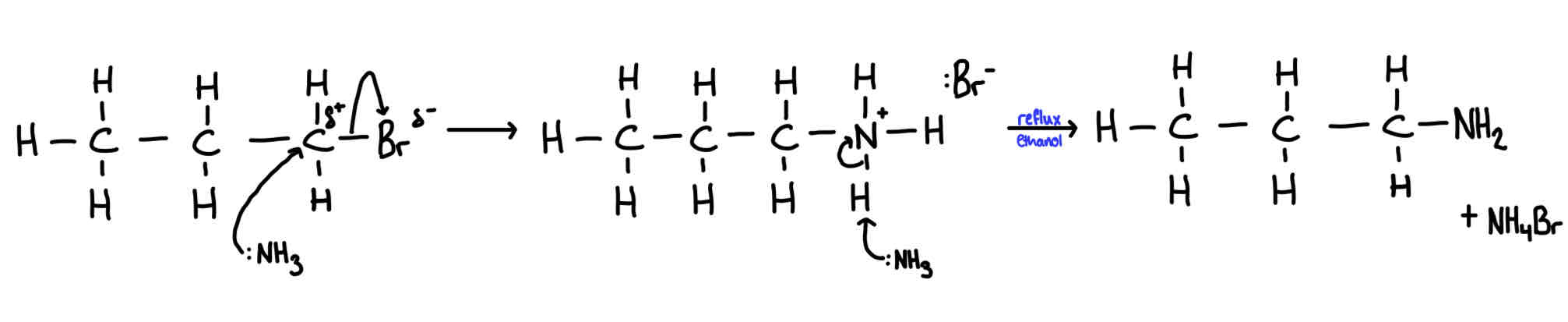
Reaction and mechanism of halogenoalkanes with ammonia
Type of mechanism: nucleophilic substitution reagent:NH3 dissolved in ethanol conditions: heat under pressure in a sealed tube
Rates of hydrolysis of primary, secondary and tertiary halogenoalkanes
Primary>Seconday>tertiary
primary hydrolyses the fastest due to it reacting via SN2 where the nucleophile directly attacks the carbon atom with the ‘exiting/leaving’ of the halogen atom.
Secondary and tertiary halogenoalkanes both have 2-3 alkyl groups attached to the carbon atoms it increases the steric hindrance making it more difficult for the nucleophile to attack.Tertiary carbocation intermediates are also the more stable than secondary making tertiary carbocations less reactive.
Rates of hydrolysis of chloro, bromo and Idoalkanes
Ido>Bromo>Chloro
Idoalkanes react the fastest as the carbon-Iodine bond is very weak due to the carbon and iodine having very similar electronegativities therefore they undergo hydrolysis quickly due to the ease of the C-I bond breaking.
Chlorine is the most electronegative between iodine and bromine therefore it undergoes hydrolysis the slowest due to the increased bond polarity between the carbon-chlorine bond making the bond more difficult to break resulting in slower hydrolysis
Trend in reactivity of primary Secondary and tertiary halogenoalkanes
primary>secondary>tertiary
Primary halogenoalkanes are the most reactive as they have the least steric hindrance around the carbon atom making it more susceptible to attack from a nucleophile.
Secondary and tertiary are the least reactive due to increased steric hindrance (more alkyl groups) around the carbon atom hinders the approach of nucleophiles.
Trend in bond enthalpy of chloro, bromo and idoalkanes
As you move down the group bond enthalpy decreases, this is nexus te size of the halogen atom increases leading to a weaker bond.
Idoalkanes have the lowest bond enthalpy and are the least electronegative they most reactive due to the polarised bond being the weakest(longer bond) and easier to break as the iodine is less attracted to the shared pair of electron.
Alcohols general formula
CnH2n+1OH
Combustion of alcohols
Alcohol + O2(g) —> CO2(g)+ H2O(l)
E.g CH3CH2OH + 3O2(g) —> 2CO2(g) + 3H2O(l)
Reaction of alcohols with PCI5
Halogenation,(chlorination) produces chloroalkanes
CH3CH2OH + PCl5 —> CH3CH2Cl + POCl3 + HCl
Can be used as a test for alcohols (-OH group) observation: misty fumes of HCl
Reaction of alcohols with sulfuric acid and potassium bromide
Produces Bromoalkanes (halogenation)
Use warmed mixture of KBr and 50% concentrated sulfuric acid
2KBr + H2SO4 —> K2SO4 + 2HBr CH3CH2OH + HBr —> CH3CH2Br +H2O
Reaction of alcohols with red phosphorus and iodine
Produces Idoalkanes (halogenation)
2P + 3I2 —> 2PI3 3CH3CH2OH + PI3 —> 3CH3CH2I + H3PO3
Why can you not react potassium iodide with sulfuric acid to produce HI
The sulfuric acid would oxidise hydrogen iodide to other products
Reaction of alcohols with potassium dichromate (vi) in sulfuric acid
Primary alcohols can be oxidised to form aldehydes, which can be further oxidised to form carboxylic acids
Conditions for carboxylic acid: use excess of dichromate,heat under reflux, distill product once reaction is finished
Partial oxidation of a primary alcohol
Primary alcohols are partially oxidised into aldehydes
Reagent: acidified potassium dichromate(K2Cr2O7), dilute H2SO4 Conditions for aldehyde: warm gently,distill out until aldehyde forms.
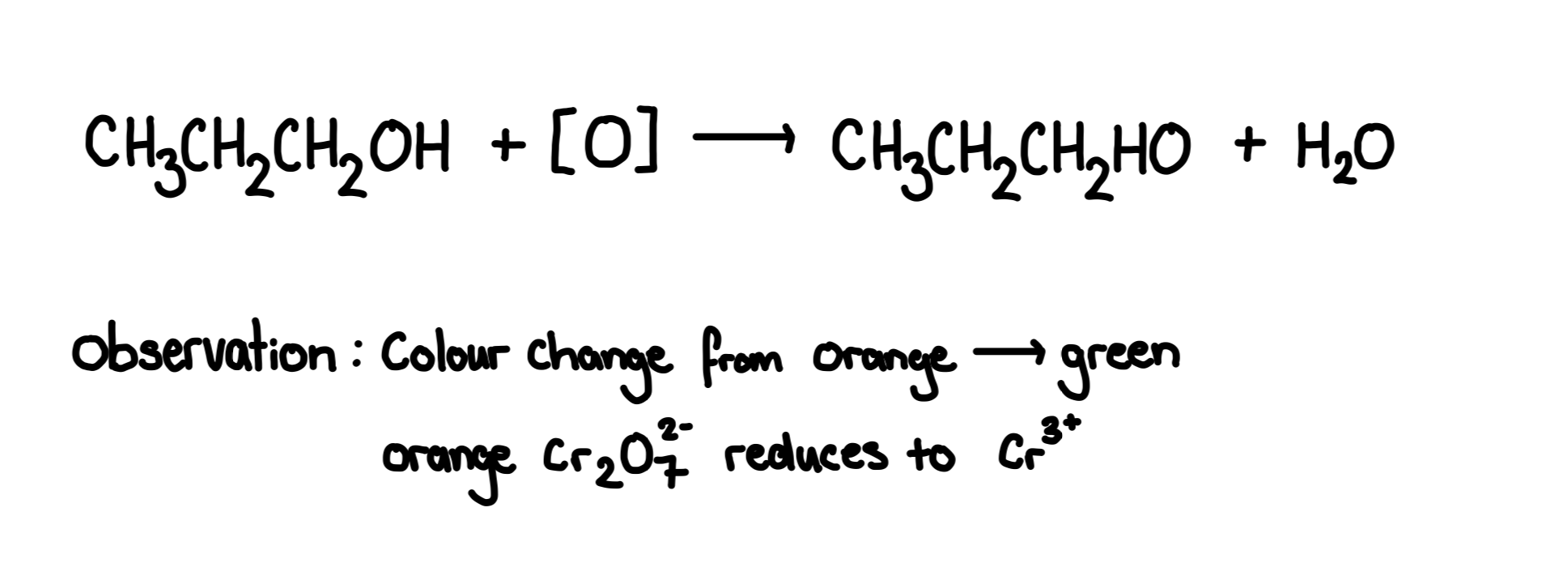
Full oxidation of primary alcohol
Primary alcohols are fully oxidised into carboxylic acids
Reagent: acidified potassium dichromate and sulphuric acid Conditions for carboxylic acid: use excess of dichromate,heat under reflux, distill product once reaction is finished
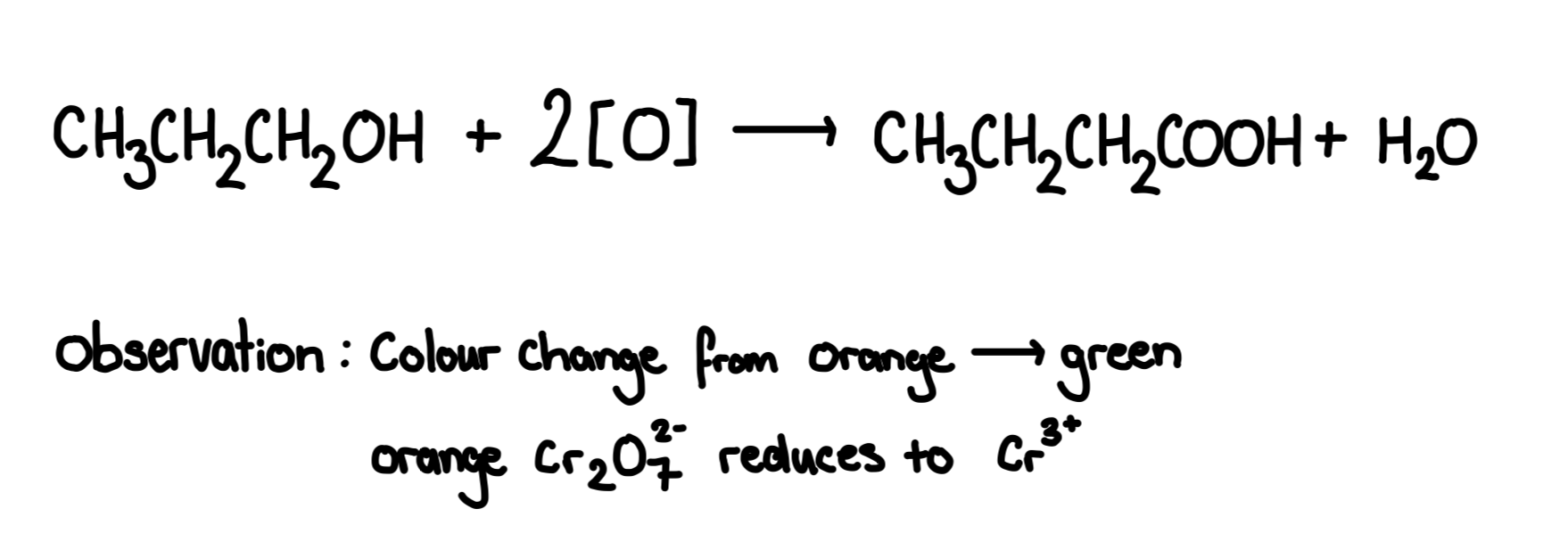
Oxidation of secondary alcohol
Secondary alcohols can be oxidised into ketones
Reagents:potassium dichromate and dilute sulphuric acid
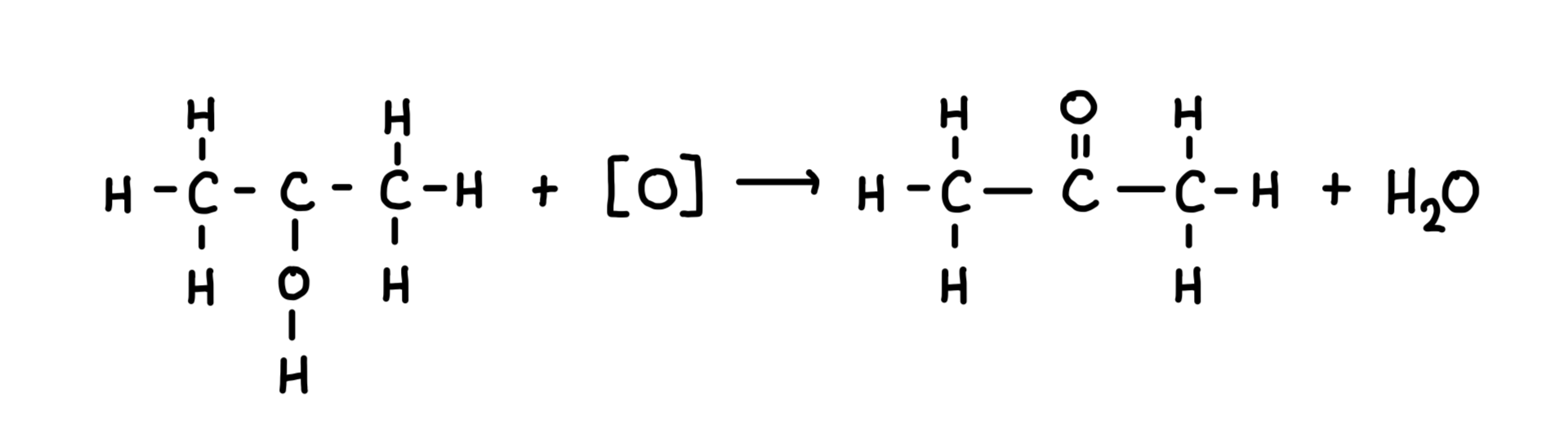
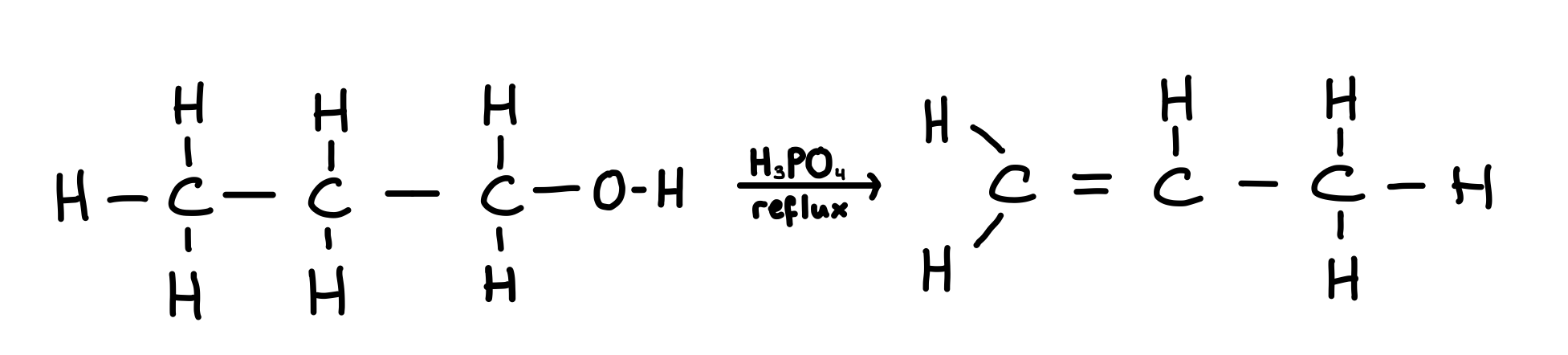
Reaction of alcohol with phosphoric acid
Alcohols react with concentrated phosphoric acid to produce alkenes in an elimination reaction
Conditions: warm under reflux Reagents: concentrated phosphoric acid (acts as a dehydrating agent-removal of water)
Why can tertiary alcohols not be oxidised
Tertiary alcohols cannot be oxidised as there is not hydrogen atom bonded to the carbon with the -OH attached to it.
This is because Oxidation creates a carbonyl (C=O) bond. The carbon on which the carbonyl bond would form is already bonded to three other carbon atoms therefore if it were to form the carbon would have 5 bonds on 1 carbon atom which isn’t possible.
Chemical test: Fhelings/Benedict’s solution
Fhelings/Benedict’s solution:blue alkaline solution containing copper (II) ions
Aldehydes have a positive test in fhelings solution as they are oxidised into carboxylic acids and copper ions are reduced to copper oxide changing the solution from blue to brick red (precipitate of copper oxide)
Ketones have a negative test as they cannot be oxidised
Chemical test : Tollens solution
Tollens reagent is an alkaline solution of Silver nitrate in excess ammonia
Aldehydes have a positive test as they are oxidised into carboxylic acids Ag+ ions are reduced into Ag atoms (forming a silver mirror)
Ketones have a negative test as they cannot be oxidised
test for carboxylic acids
The addition of sodium carbonate in fhelings/Benedict’s solution will cause fizzing as carbon dioxide is produced
Heating under reflux
Distillation
Drying with an anhydrous salt
Boiling temperature determination