covalent bonding 2
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
what is electronegativity
a measure of the tendency of an atom to attract a bonding pair of electrons
in what example are electrons shared equally between two atoms
diatomic molecules have equal tendencies to attract the covalently bonded electron pair and there is no difference in electronegativity
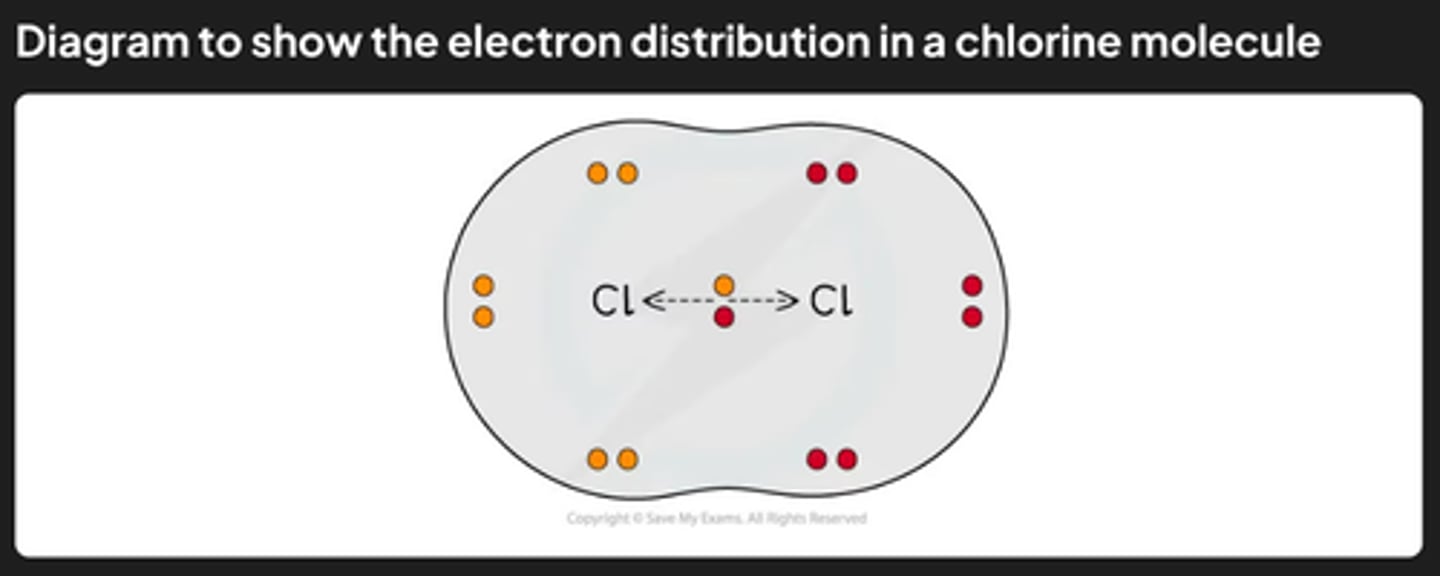
how to remember the diatomic molecules
BrINClHOF
are covalent bonds polar or non-polar
they can be both
why can covalent bonds be both polar or nonpolar
if there is any difference of electronegativity the bond is polar, since covalent compounds can have differences of electronegativity they can be polar
covalent compounds which are non polar can come from either diatomic molecules or cancelling out of dipoles
what is a polar bond
when electrons are distributed unequally within a bond so one side with be delta positive and the other will be delta negative
what does the polarity of the bond depend on
the difference of electronegativity between the two atoms
the higher the difference of electronegativity....
the more polar the bond
what the actual frick is a dipole then
a dipole is a measure of how polar a bond is (how high the difference of electronegativity between the atoms in a bond)
how is a dipole represented and which way does the arrow go
the arrow points to the delta negative side of the bond/ the more electronegative side

how to determine whether a molecule is polar
have to look where the dipoles are in a molecule
(unless diatomic they will always have dipoles)
and see if the shape cancels the dipoles out
what direction should two dipoles be in relation to one another to be classed as 'cancelled out'
away from each other
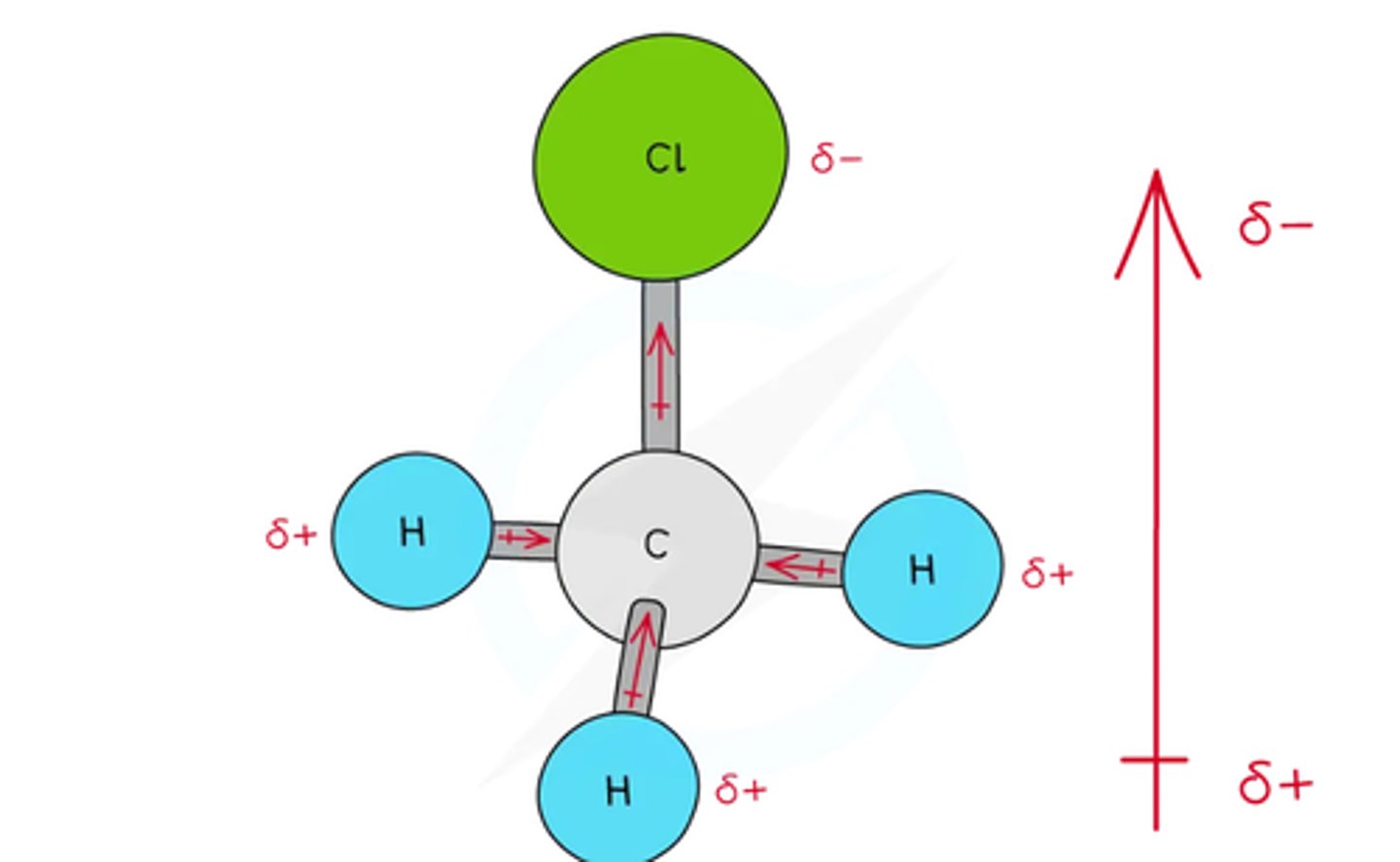
is this polar or non polar ->
POLAR BECAUSE THEY DONT CANCEL OUT BECAUSE THEYRE GOING TOWARDS EACH OTHER
is this polar or non polar ->
NON POLAR BECAUSE THEY CANCEL OUT BECAUSE THEYRE GOING AWAY FROM EACH OTHER
is co2 polar or non polar
draw lewis diagram
nonpolar as its dipoles cancel out as theyre going away from each other
is NH3 polar or nonpolar
polar, dipoles do not cancel out and even if they did the lone pair affects the shape of the molecule
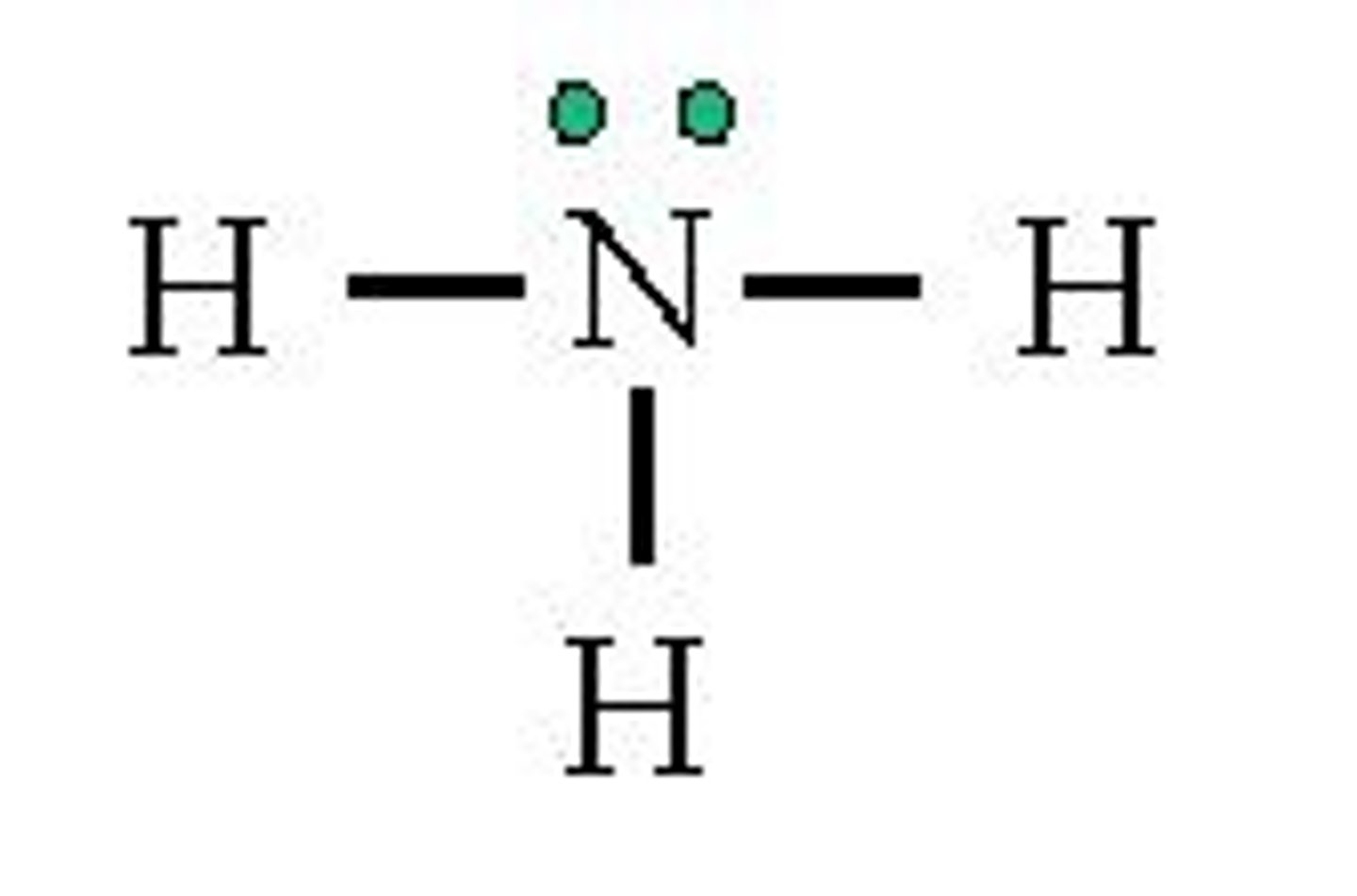
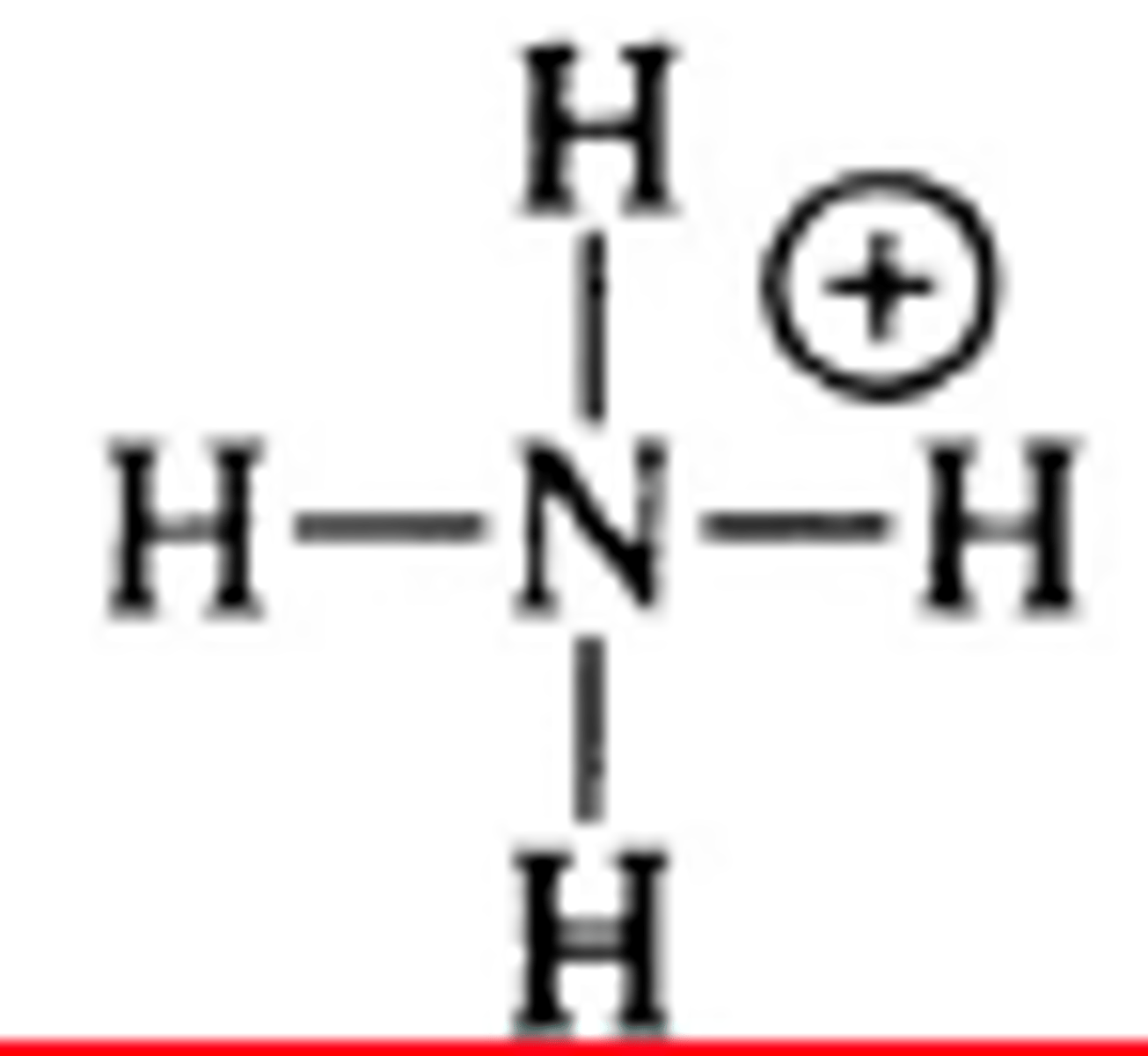
NH4 polar or nonpolar
nonpolar but not because the bonds cancel out because they dont but instead because its symetrical
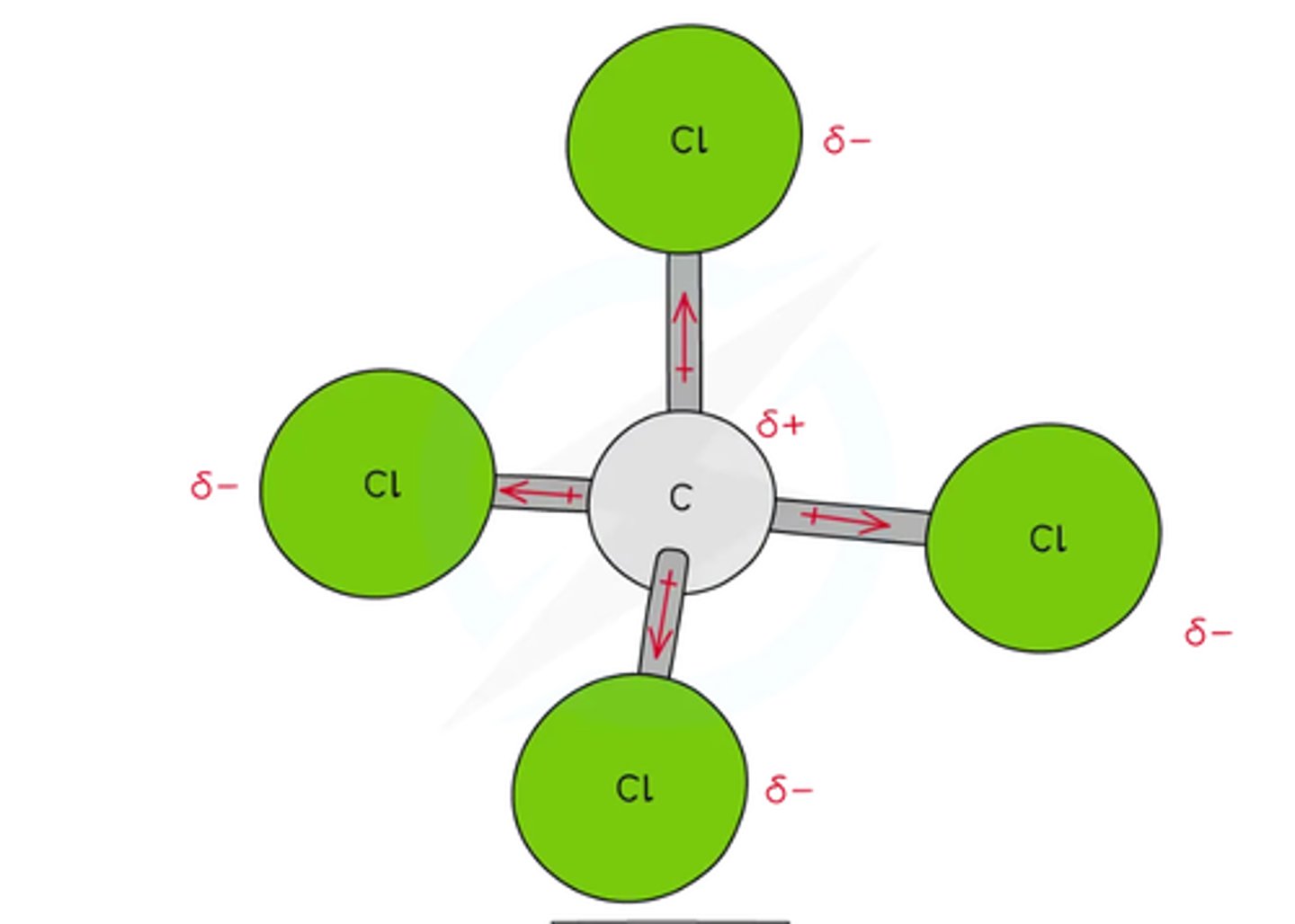
CCl4 polar or nonpolar
non polar becuse the dipoles cancel out
what are the ATOMS within a single simple molecule held together by
covalent bonds
how are the different MOLECULES in simple molecular structures held togther
if you said covalent bonds close the laptop and jump off a cliff because youre actually cooked
its intermolecular forces between the MOLECULES and covalent bonds between the atoms within the molecules
what is really really really REALLY important to know about intermolecular forces
they occur between the simple covalent molecules to hold those together unlike the giant covalent which are made held together
what are the 3 types of intermolecular forces
london dispersion forces (instantaneous dipole induced dipole)
dipole induced dipole
dipole-dipole interaction
hydrogen bonding
what are the weakest of the intermolecular forces
london dispersion forces
what contains london dispersion forces
literally every single molecule ever created ever on this earth ever has london dispersion forces
what are london dispersion forces
they are temporary/instantaneous dipole induced dipoles where one atom becomes temporarily a dipole causing adjacent atoms to be attracted/repelled
how does a london disperion force come about -how can everything including nonpolar bonds have a temporary dipole
well because electron motion is constant within atoms, so if too many electrons move to one side even for a second it makes a dipole which makes delta positive side and delta negative side so adjacent atoms are either attracted or repelled which makes a dipole in that atom causing attraction/interaction
although london dispersion forces are just weak (like will) they can still vary in strength what are the two things that affect london dispersion forces strength
surface area
number of electrons
how does the number of electrons affect london dispersion forces
the more electrons the greater the likelihood of temporary dipole the MORE FREQUENT, and the greater the magnitude of the temporary dipole
what can we compare to show the strength of intermolecular forces
energy needed to boil and vaporisation
the stronger the intermolecular force
the higher the energy needed to boil and vaporise it
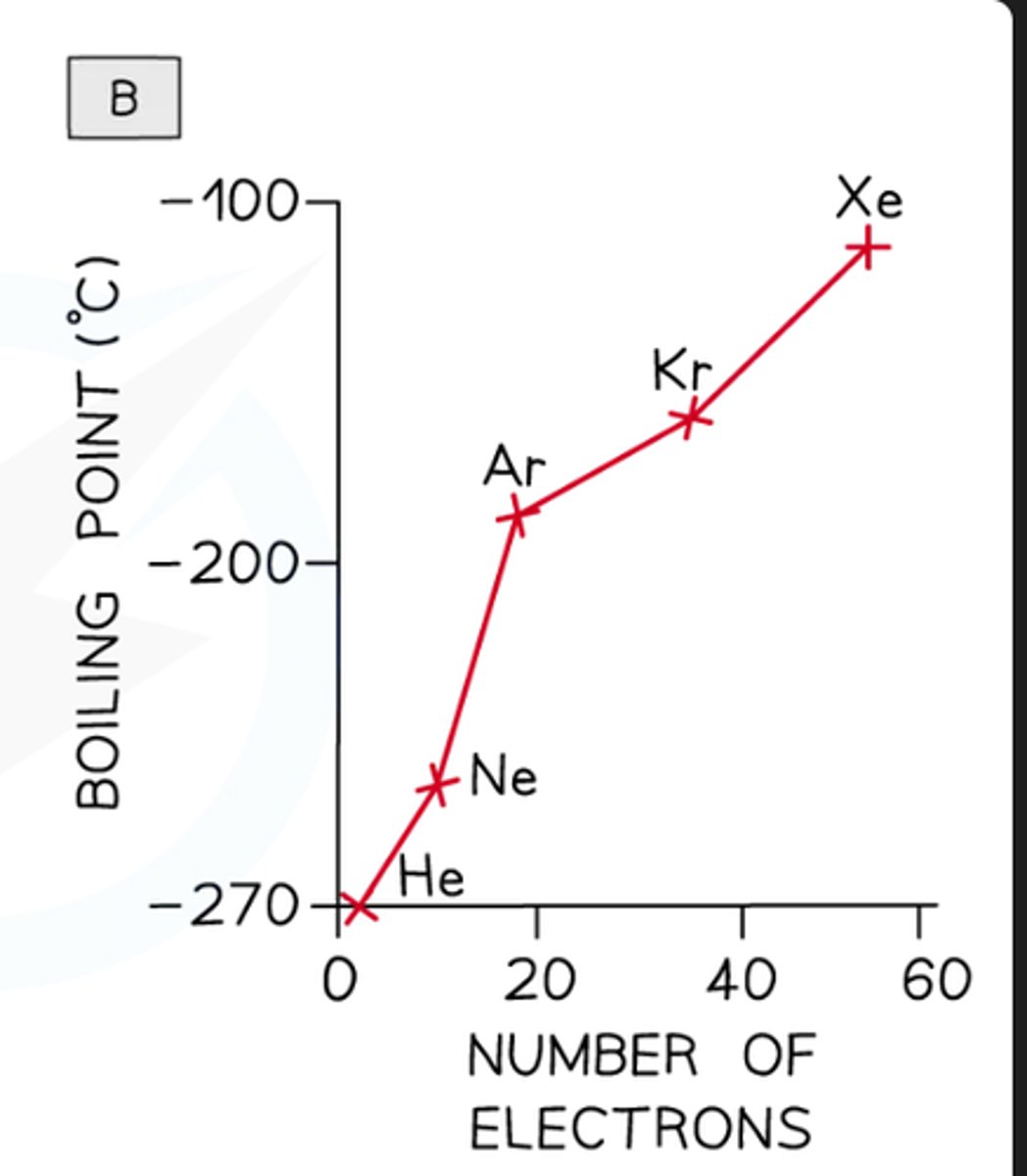
how to compare the effect of no of electrons on london dispersion forces
go down the noble gas group (has to be noble gases because they all only have london dispersion) and measure bp
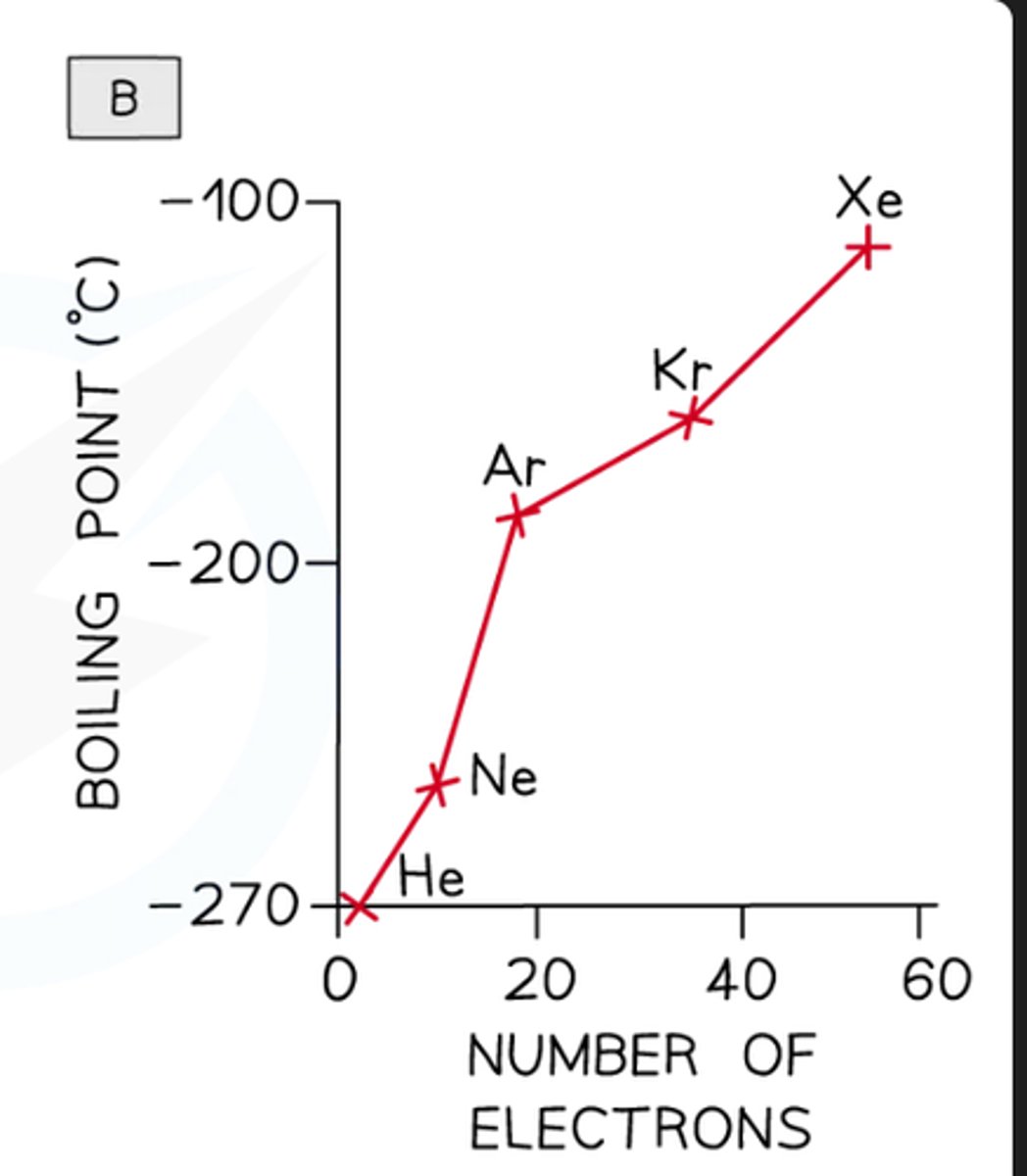
what do you HAVE to be careful of
which type of intermolecular force is in which
they ALL have london dispersion forces but some have other forces too like hydrogen bonds or dipole-dipole etc so it will affect it
explain why the bp increases down group 8
more electrons so more frequent temporary dipoles with ahigher magnitude so stronger london dispersion forces
how can surface area affect the strength of london dispersion forces
more surface area of a molecule the more interaction itll have with adjacent molecules the greater the ability to induce another dipole when it becomes an instanteaous dipole so the greater the london forces
how can you compare surface area affect on london dispersion forces
using ISOMERS because theyre molecules with the same atoms but arranged differently
explain dipole induced dipole interactions
permanent dipoles can cause temporary dipoles in nonpolar substances
acts in addition to london dispersion
example of when you would see dipole induced dipole
mixture of polar and nonpolar molecules e.g HCl H-Cl (polar) is a dipole causing Cl-Cl (nonpolar) to become a temporary dipole
explain DIPOLE-DIPOLE interactions
when a permanent dipole is attracted or repelled by the delta positive or delta negative side of another permanent dipole
acts alongside london dispersion forces
what can you compare to prove dipole-dipole interactions are stronger than london dispersion forces and why
propanone and butane because
-they have the same number of electrons so wont affect the london dispersion forces
-butane is non polar so it will only have london dispersion forces
-propanone is polar so will have dipole-dipole interaction
compare propane and butane
due to the dipole-dipole interaction in propanone because of its polar character it has a higher boiling point as more energy is needed to break the bonds between the interactions then in butane because butane is nonpolar and only consists of london dispersion forces which do not require as much energy to break
order of strength of intermolecular forces
london dispersion, dipole induced dipole, dipole-dipole, hydrogen bonds
what does hydrogen atom have to BOND with for hydrogen bonding MY FAVE ACRONYM EVER (back to atoms apparently)
ONF
Oxygen
Nitrogen
FLourine
why those three
because it gives the greatest difference in electronegativity because they are the most electronegative substances -strongest bond for a reason am i right
strongest type of intermolecular force
hydrogen bonding
what are the 4 main giant covalent structures you need to know
graphite/graphene
diamond
buckminster fullerene aka c60 fullurene
silicon/silicon dioxide
what are all giant covalent molecules in relation to carbon
they are all allotropes of carbon except silicon dioxide
meaning they are made of entirely carbon
structure of diamond
1. tetrahedral structures
2. bond angle 109.5
bonding in diamond
1. each carbon covalently bonded to 4 others forming tetrahedral
2. bond angle of 109.5
structure in diamond
each carbon covalently bonded to 4 others forming tetrahedral
(diamond is pretty boring, comparing it would mainly be 'and diamond doesnt' ifykwim)
uses of diamond
jewellery and cutting tools
bonding in graphite
-each carbon is COVALENTLY bonded to 3 other carbons to form a layer of hexagonal rings
-bond angle 120
-delocalised electron free to move and carry a charge
structure of graphite
-each carbon covalently bonded to 3 other carbons forms layer of hexagonal rings
-bond angle 120
-intermolecular forces holding the layers together
-delocalised electrons free to move and carry a charge
bonding of buckminster fullerene
made up of 60 fullernes
each carbon COVALENTLY bonded to 3 others
delocalised electrons
bond angle 120
structure of buckminster fullerene
football shaped
made up of pentagonal and hexagonal rings
semi-conductor due to delocalised electrons free to move and carry a charge
bonding of silicons in just silicon and the structure
each silicon is covalently bonded to 4 other silicons
tetrahedral silicon structure with bond angle of 109.5
similarities between all covalent lattice structures
they all have high mp/bp, require lots of energy to break the bonds
insoluble in water
physical differences
graphite can be soft (layers slide yk)
diamond and silicon very hard
what are van der vaals forces specific to
everything but hydrogen bonding
london dispersion
dipole-dipole
dipole induced dipole
when asked to draw two different hydrogen bonding interactions
look for a link between hydrogen and ONF
but then also there will most likely be a hydrogen on the other which can link ONF in the same way
e,g CH3OH and H20
the h on ch3oh can link to the o on h20
but also the h on h20 can link to the o on ch3oh you feel me
2 requirements for hydrogen bonding
1. hydrogen must be bonded to O N or F to be electronegative enough to form a bond to another molecule
2. there must be lone pairs on the O N or F
this must happen on both molecules if they are different
can two methanals CH2O form hydrogen bonds together
no
lone pairs on oxygens yep
but the hydrogen is not bonded to the O have to draw out lewis structures to figure out
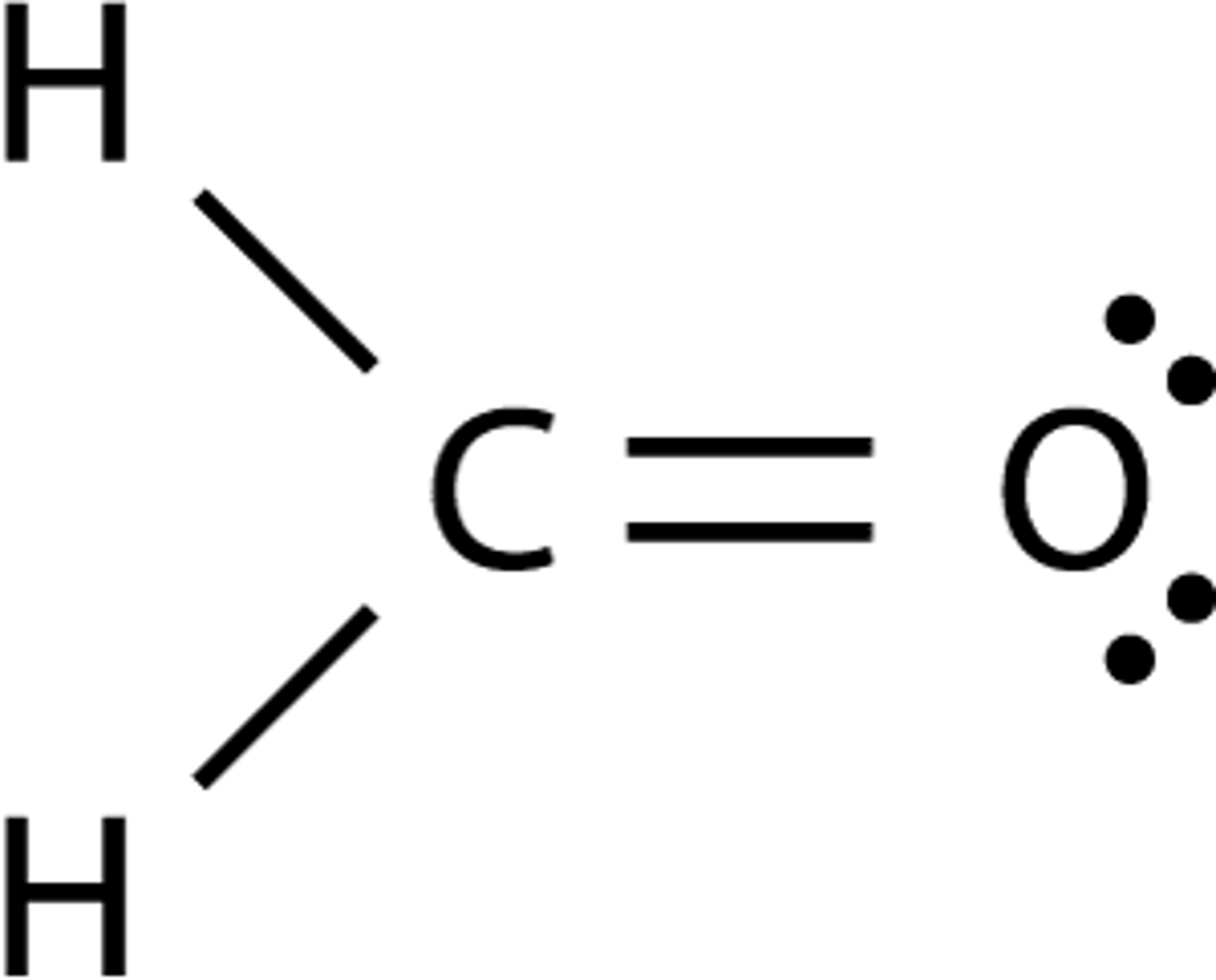
can methanoic acid CH3OH form hydrogen bonds with water H2O
yes because methanoic acid has a hydrogen bonded to ONF and ONF has lone electrons
water also has a hydogen bonded to ONF and ONF has lone electrons
both ONF is oxygen in this case