Strong Acids & Strong Bases + Solubility Rules
0.0(0)
0.0(0)
Card Sorting
1/11
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
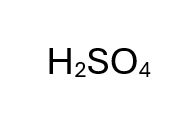
H2SO4
Sulfuric Acid - Strong Acid
2
New cards

HBr
Hydrobromic Acid - Strong Acid
3
New cards
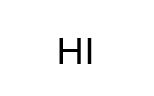
HI
Hydroiodic Acid - Strong Acid
4
New cards
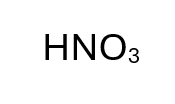
HNO3
Nitric Acid - Strong Acid
5
New cards

HCl
Hydrochloric Acid - Strong Acid
6
New cards

HCLO3
Chloric Acid - Strong Acid
7
New cards

HCLO4
Perchloric Acid - Strong Acid
8
New cards
Bases with Group 1 Elements are ______
Strong Bases
9
New cards
Group 2 Elements that From Strong Bases
Sr, Ba, Ca
10
New cards
Amines
Bases ending in -NH, -N, -NH2
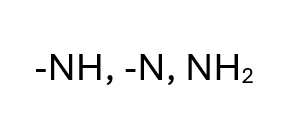
11
New cards
Solubility Rules
All Group 1 Elements are Soluble
All Nitrates, Chlorates, and Acetates are Soluble
All Ammonium Compounds are Soluble
12
New cards
All Strong Acids
HCl
HBr
HI
HClO3
HClO4
H2SO4
HNO3