MCAT Organic Chemistry - Spectroscopy
1/38
Earn XP
Description and Tags
500
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Spectroscopy
measures the energy differences between the possible states of a molecular system by determining the frequencies of electromagnetic radiation absorbed by the molecules
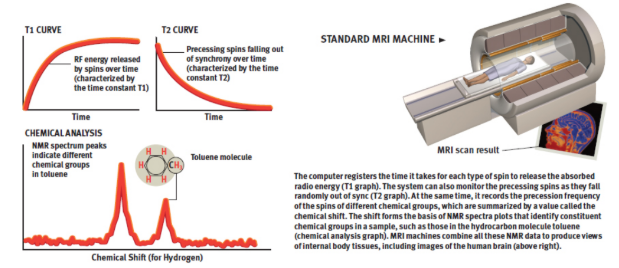
magnetic resonance imaging (MRI)
measure 1H–NMR spectra of water molecules in different environments in the body; multiple cross-sectional scans of the patient’s body are taken, and the various chemical shifts of absorbing protons are translated into specific shades of grey which produces a picture that shows the relative density of specific types of protons
dark area = waters
light area = fattier tissue
Infrared (IR) spectroscopy
measures molecular vibrations, which helps estimate types of bonds; infrared light is passed through a sample, and the absorbance is measured; for an absorption to be recorded, the vibration must result in a change in the bond dipole moment
range: 2500 to 25,000 nm = 4000 to 400 cm−1
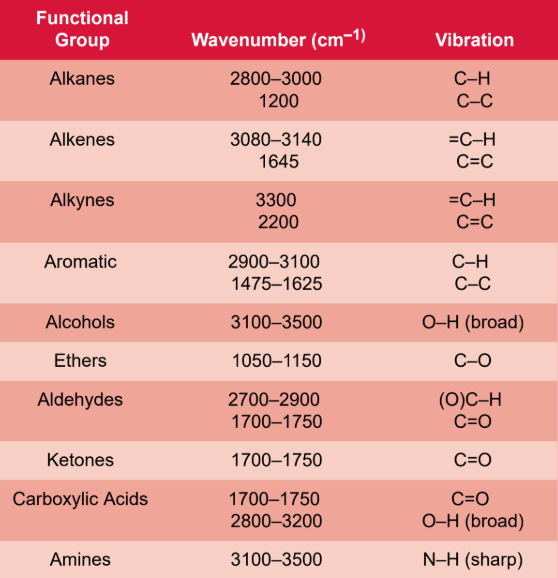
infrared light range
700 nm to 1 mm
wavenumber
analog of frequency
= 1/λ
units: cm−1
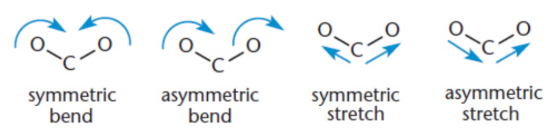
molecular vibration
bond stretching, bending, or combinations of different vibrational modes; include twisting and folding; Symmetric stretches do not show up in IR spectra because they involve no net change in dipole movement
fingerprint region
complex vibration patterns, caused by the motion of the molecule as a whole, in the 1500 to 400 cm−1 range; the specific absorbance pattern is characteristic of each individual molecule
hydroxyl group (O−H) IR
broad (wide) peak
3300 cm−1 for alcohols
3000 cm−1 for carboxylic acids (carbonyl pulls electron density → shifts absorption lower)
carbonyl group (C=O) IR
sharp (deep) peak
around 1700 cm−1
amine group (N−H) IR
same region as O−H bonds, but have a sharp peak
around 3300 cm−1
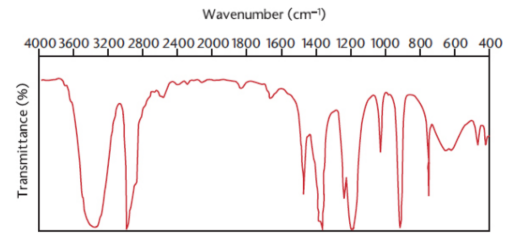
transmittance
the amount of light that passes through the sample and reaches the detector
IR spectra
plotted as percent transmittance vs. wavenumber; maximum absorptions appear as the bottoms of valleys on the spectrum
ultraviolet-visible (UV-vis) spectroscopy
passing ultraviolet light through a sample that is usually dissolved in an inert, nonabsorbing solvent, and recording the absorbance, caused by electronic transitions between orbitals; wavelength of maximum absorbance, which tells us the extent of conjugation within conjugated systems
UV spectra
plots absorbance vs. wavelength
HOMO–LUMO gap
energy gap between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO); lower energy gap = longer wavelengths = more conjugation = more excitable
Conjugation
molecules with unhybridized p-orbitals, can be excited by ultraviolet light; shifting the absorption spectrum, resulting in higher maximum wavelengths; larger conjugated molecules may even absorb light in the visible range → coloured compounds
Nuclear magnetic resonance (NMR)
certain atomic nuclei have magnetic moments that tend to align either with or against the direction of an applied field; irradiated with radiofrequency pulses that match the energy gap between the two states, exciting some nuclei depending on an atom’s magnetic environment
α-state
Nuclei with magnetic moments that are aligned with the magnetic field; lower energy
β-state
Nuclei with magnetic moments that are aligned against the magnetic field; higher energy
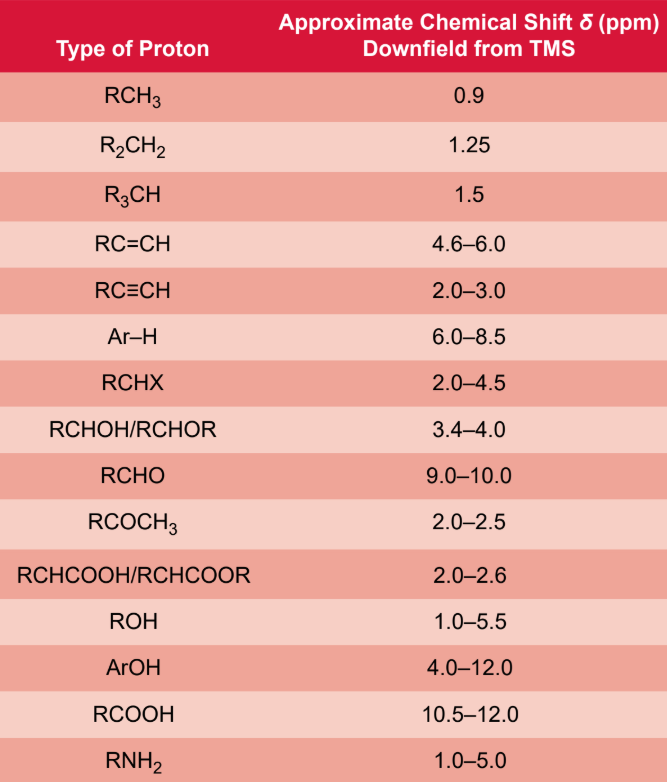
chemical shift (δ)
standardized method of plotting the NMR spectrum using an arbitrary variable with units of parts per million (ppm) of spectrometer frequency
NMR spectra
plot of frequency vs. absorption of energy
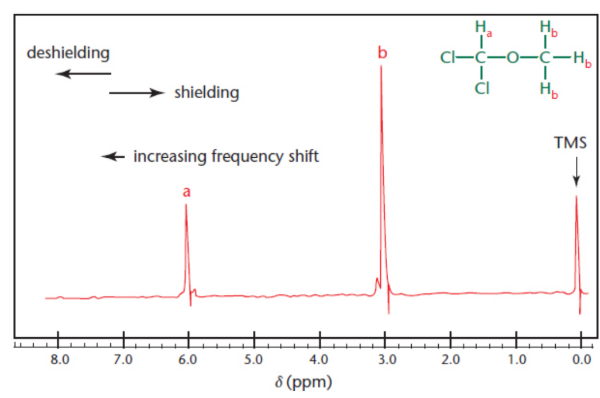
downfield
towards a larger chemical shift; increases to the left; signals become relatively deshielded
tetramethylsilane (TMS)
calibration standard/reference peak to mark 0 ppm
chemically equivalent
protons that have the same magnetic environment; lead to the same peak
integration
the area under the peaks; the ratio of different peaks corresponds exactly to the ratio of protons that produced each peak
deshielding
electron density away from the proton, leaving it more vulnerable to the magnetic field
spin–spin coupling (splitting)
two protons in such close proximity to each other that are not magnetically identical; the magnetic environment of one group of protons can be affected by another
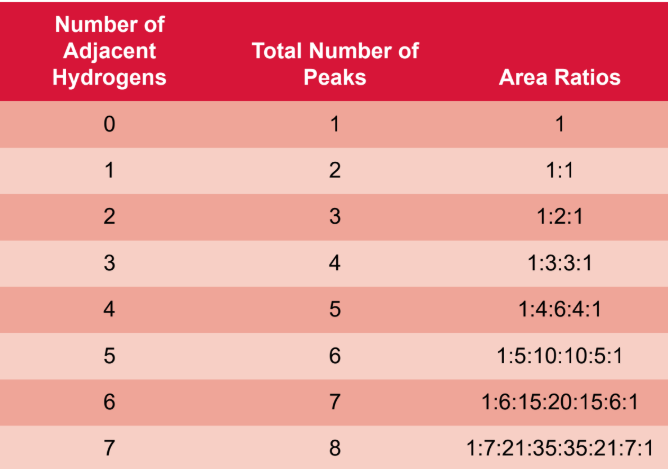
n + 1 rule
if a proton has n protons that are three bonds away, it will be split into n + 1 peaks
coupling constant (J)
magnitude of this splitting, measured in hertz
multiplet
peaks that have more than four splits
methyl group (-CH3) 1H-NMR
0.9 ppm
methylene group (-CH1) 1H-NMR
1.25 ppm
methine gorup (-CH) 1H-NMR
1.5 ppm
aldehyde ((C=O)-H) 1H-NMR
9 - 10 ppm
carboxylic acid ((C=O)-OH) 1H-NMR
10.5 - 12 ppm
aromatic hydrogen (Ar-H) 1H-NMR
6.0 - 8.5 ppm
sp3 hybridized carbons 1H-NMR
0.0 to 3.0 ppm
sp2 hybridised carbons 1H-NMR
4.6 - 6.0 ppm
sp hybridised carbon 1H-NMR
2.0 - 3.0 ppm