O chem midterm lab
1/77
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
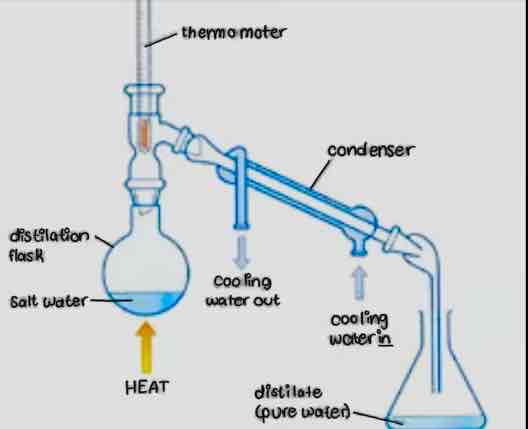
What is distillation? What is this technique useful for?
Separation of multiple liquid components based on their different boiling points. The technique is useful for separating a liquid mixture when the components have different boiling points.
What are the two process of distillation?
Evaporation and Condensation
What is a simple distillation?
It is separating a liquid mixture when the components have different boiling points or when one of the components will not distill.
What are the two cases where it is possible to get a good separation by using a simple distillation?
1. if the boiling points A and B differ by a large amount (>100), and if the distillation is carried our carefully, it will be possible to get a fair separation of A and B
2. If A contains a fairly small amount of B (<10) a reasonable separation of A from B can be achieved.
What is fractional distillation? When it should be used
Fractional distillation = a process that has the effect of many simple distillations.
It must be used when the boiling point differences of components to be separated are not large (usually by less than 40)
Be able to identify the following parts on a simple & fractional distillation apparatus: distilling flask, distilling head, fractional distillation column, condenser, vacuum adapter, and receiving flask.
Purpose of adding boiling chips
Boiling chips prevent the mixture from bumping when heated. Boiling chips provide a nucleation surface for when bubbles form and ensure a smooth boiling process. Adding boiling chips before heating prevents the liquid from boiling over and out of the apparatus.
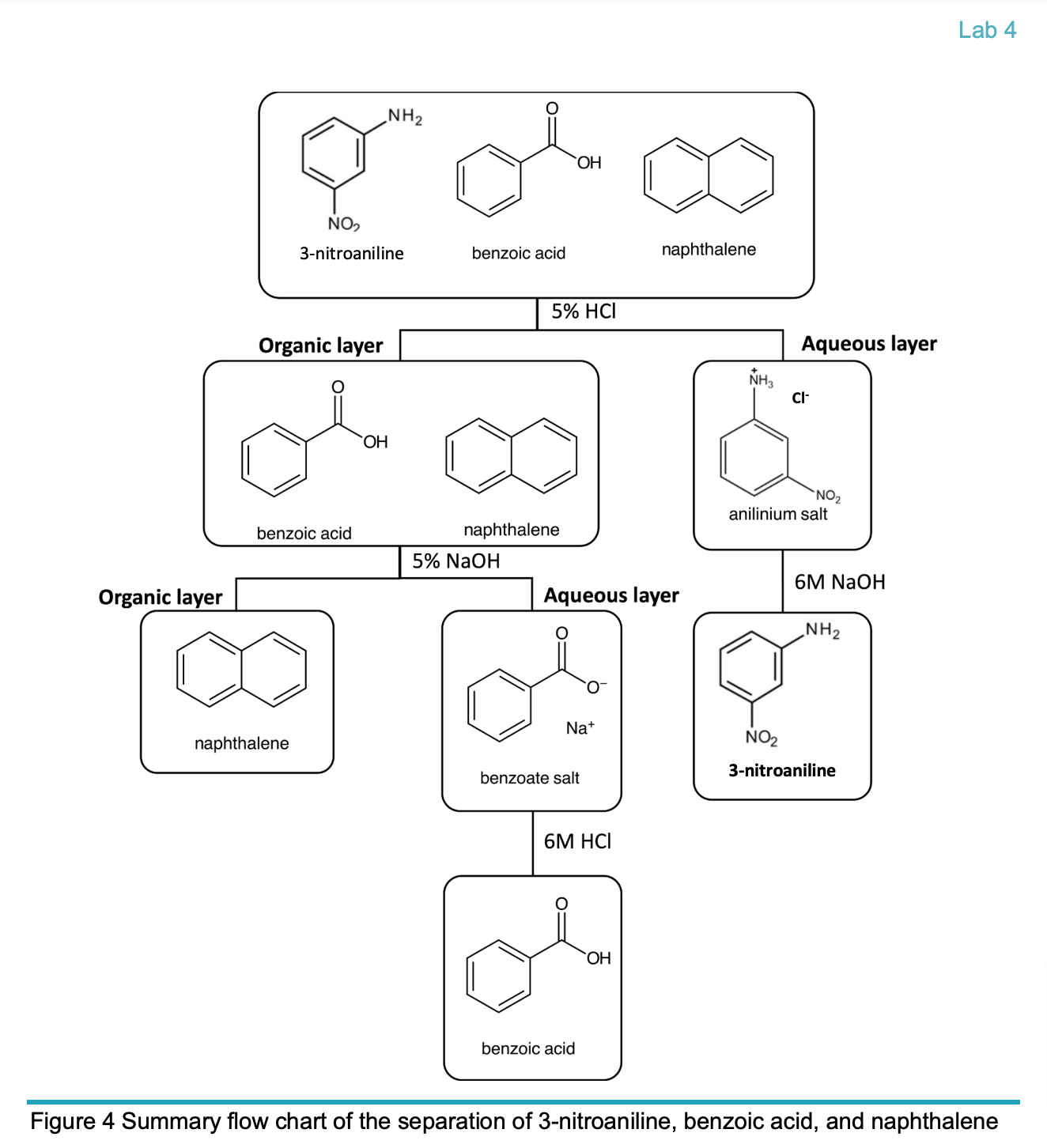
Summary flow chart of the separation of 3-nitroaniline, benzoic acid, and naphthalene
In a separatory funnel which layer is top layer and which layer is bottom layer, why?
Separation into 2 layers –depends on densities of the solvents
Lower density solvent always on top layer
higher density at lower layer
How can you separate an organic acid and an organic base using separatory funnel?
By opening the stopcock at the bottom of the separatory funnel and allowing one layer to flow out
Percentage recovery calculation
Amount of substance recovered / Initial amount of substance) x 100%
(Initial mass - left over mass)/Initial massx 100
EXMPL. Let's say you calculate the theoretical yield of a reaction to be 10.0 grams of product. After performing the experiment, you obtain a mass of 8.0 grams of product. The percent recovery would be: (8.0 g / 10.0 g ) x 100 = 80% recovery.
What are two key points to know about crystallization?
1. It is wasteful
2. A crystallization is successful only if there is a small amount of impurity
Which property is the basis of recrystallization?
Solubility is the property that is the basis of recrystallization.
How melting points can be used to assess the purity of a mixture?
Melting points can be used to assess the purity of a mixture because a pure substance has a closed, narrow melting point range, while an impure substance melts over a broader and lower temperature range. The more impurities present, the more the melting point decreases and broadens.
How does the addition of an impurity affect the melting point?
When we add impurities to the pure substance, the melting point of solid is decreased because impurities weakens the lattice structure of solids due to which it become less stable and melts before its original melting point. This is called melting point depression.
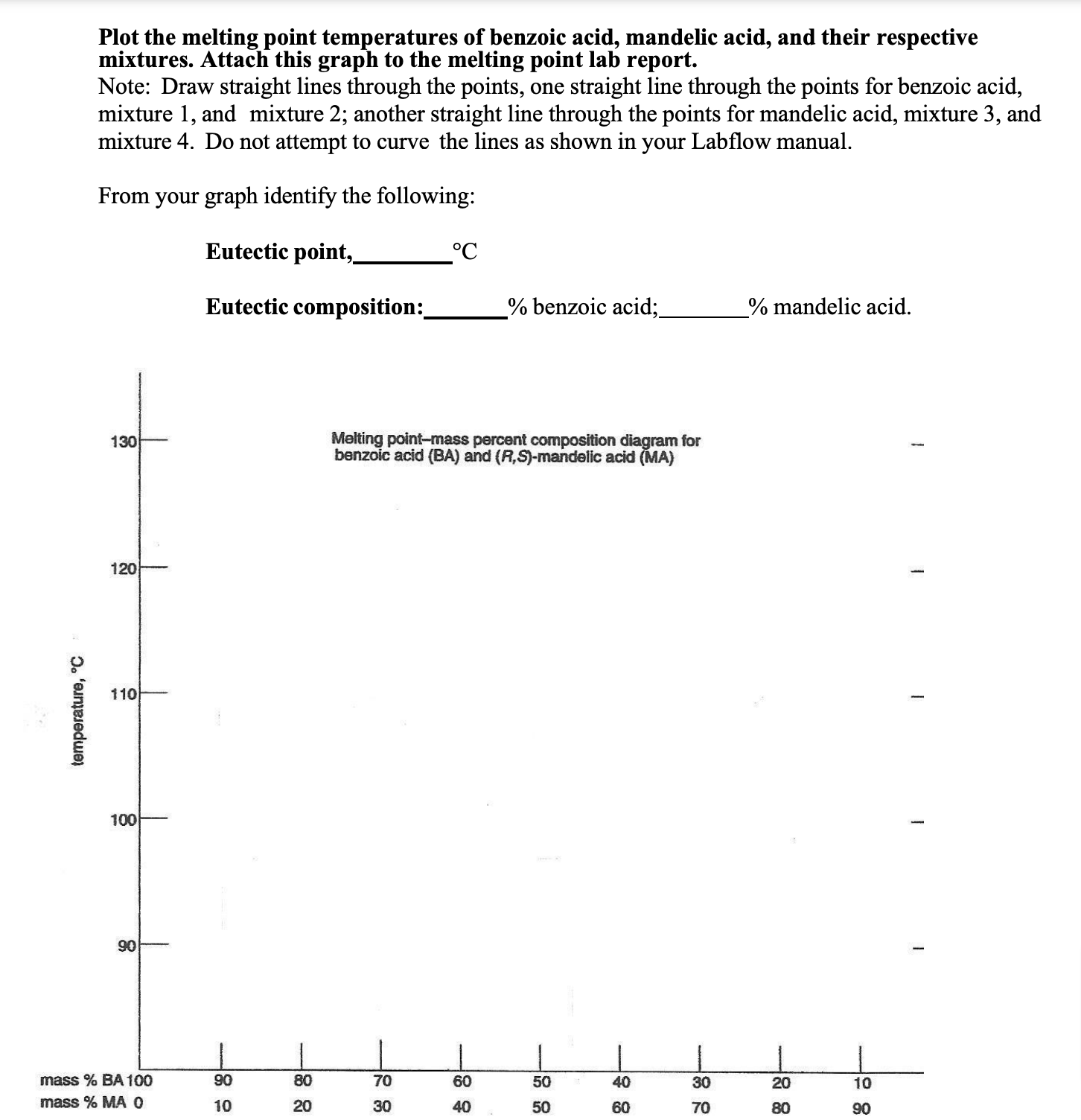
Know where to identify the melting points of each component of a mixture and the eutectic point on a melting point-composition curve
On a melting point-composition curve, the melting points of each component of a mixture are found at the extreme left and right points of the curve, representing 100% of each pure component, while the eutectic point is the lowest point on the curve
Rf value calculation
Ref = distance traveled by the compound divided by or over the distance traveled by the convent front
EXMPL = if a compound travels 1.5 cm and the solvent front travels 6.0 cm, the Rf value is 0.25.
A student extracted 300 mg crude caffeine from 400 mg vivarin tablets. On recrystallization, the student reported 150 mg pure crystalline Caffeine. Calculate the percentage recovery of the crude and recrystallized Caffeine based on vivarin tablets.
It is safe in the organic chemistry lab to add boiling chips to an already boiling liquid. (True/False)
“Common sense” false
If a mixture contains two compounds with one being more volatile (more than 70°C) than the other, then distillation would be preferred.
Simple
Close Boiling Points: If the boiling points of the two components are close, the separation won't be effective with simple distillation.
Fractional Distillation: Instead, fractional distillation is used, which employs a fractionating column to provide multiple vaporization-condensation cycles, allowing for better separation of components with similar boiling points.
When mixing reagents using a separatory funnel one should not vent to ensure there is no spill
False.
When using a separatory funnel, it is actually important to vent the funnel periodically while mixing reagents due to pressure build up and safety reasons.
A drying agent is an insoluble, anhydrous organic salt
True
Common examples include magnesium sulfate (MgSO₄), sodium sulfate (Na₂SO₄), and calcium chloride (CaCl₂). These substances work by absorbing water, effectively drying the mixture
Which of the following are valid reasons as to why gentle shaking is necessary during the extraction of caffeine from?
the formation of emulsions does not occur
to prevent the excessive buildup of pressure inside the separatory funnel
In this experiment, Caffeine is extracted and purified by the process of.
recrystallization
Which of the following best identifies why you should not heat organic solvents over a Bunsen burner flame?
most organic solvents are flammable and would ignite if exposed to an open flame
Which of the following are useful functions served by knowing the melting point of an organic compound?
identify the compound
assess the purity
From Experiment 2, “Separation of benzoic acid, 3-nitroaniline, and naphthalene”, the three compounds belonging to the chemical classes -------------, --------------, and ---------------.
Benzoic Acid: This is an aromatic carboxylic acid. It has a carboxyl group (-COOH) attached to a benzene ring.
3-Nitroaniline: This compound is an aromatic primary amine. It has an amino group (-NH₂) attached to a benzene ring, and the nitro group (-NO₂) is also attached to the benzene.
Naphthalene: This is an aromatic hydrocarbon. It consists of two fused benzene rings.
What is the best listed approach for viewing spots that are not visible on a TLC plate? Select one:
UV light:
Sensitivity
Fluorescent indicators
Non destructive
Detection of compounds
Why is a finely powered sample preferred to be used during a melting point measurement?
B/c it helps to ensure a rapid and efficient transfer of heat through the entire sample
What is the purpose of using drying agent in an organic reaction?
To absorb small amounts of water from organic solution.
Common Drying Agents:
Anhydrous Sodium Sulfate (Na₂SO₄): A widely used drying agent for organic solvents.
Magnesium Sulfate (MgSO₄): Another common drying agent, particularly effective for drying organic liquids.
Calcium Chloride (CaCl₂): Used for both liquid and gas drying, it can absorb water from the atmosphere as well.
Calculate the Rf value for a spot in a TLC experiment if the solvent moved 14.7 cm and the spot moved 8.2 cm from the origin.

A value that quantifies the distance traveled by a substance relative to the distance traveled by the solvent
Rf:
(Retention factor) is the value that quantifies the distance traveled by a substance (the spot) relative to the distance traveled by the solvent front in a chromatography experiment, such as thin-layer chromatography (TLC). It is calculated using the formula:

Before adding a sample or solvent to a separatory funnel, what should you have in place? Select one or more:
A stopcock in the closed position.
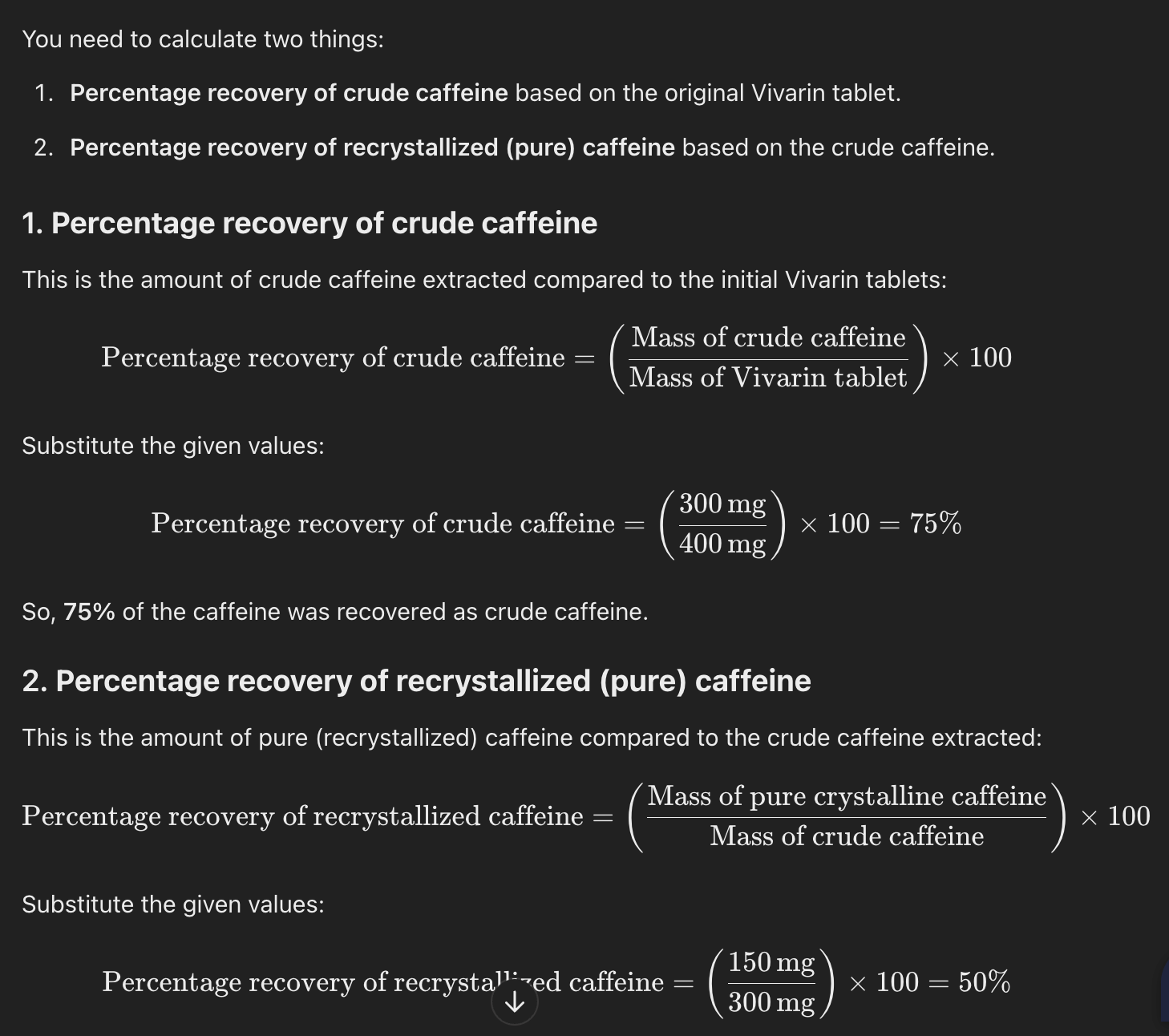
From this diagram of a microscale simple distillation set-up (diagram not shown), identify the purpose of each labelled part.
Distillation is the separation of multiple ____ components based on their different ____.
As the mixture is heated and the first component ____, its ____ form travels through the distillation set-up and ____ into a different container.
Liquids & Boiling Points
Boils & Vapor & Condenses
To insert a thermometer into an adapter, use ____ to prepare the thermometer. Then, hold the thermometer ____ the adapter and the thermometer into the adapter.
Mineral Oil - Close to - Slowly Turn
What are the concerns presented by overheating a distillation to a dry flask?
The remaining solid residue might contain explosive peroxides.
The empty glassware might heat quickly, igniting vapors from the distillation.
Before turning on the heat for a microscale distillation, what should you confirm about the set-up?
Secure connections at the joints
Existence of some opening in the set-up
Presence of boiling chip in the sample
Where should the tip of the thermometer be placed in a microscale distillation set-up?
At or slightly below the side arm fo the distillation head
An azeotrope is a mixture that has ____ composition in the ____ & ____.
Therefore, an azeotropic mixture ____ be separated by distillation.
The Same - Gas & Liquid Phase - Cannot
Suppose you are using distillation to separate cyclohexane and toluene. The boiling point of cyclohexane is ____ oC and the boiling point of toluene is ____ oC.
Therefore, the liquid collected first should be ____.
81 - 111 - Cyclohexane
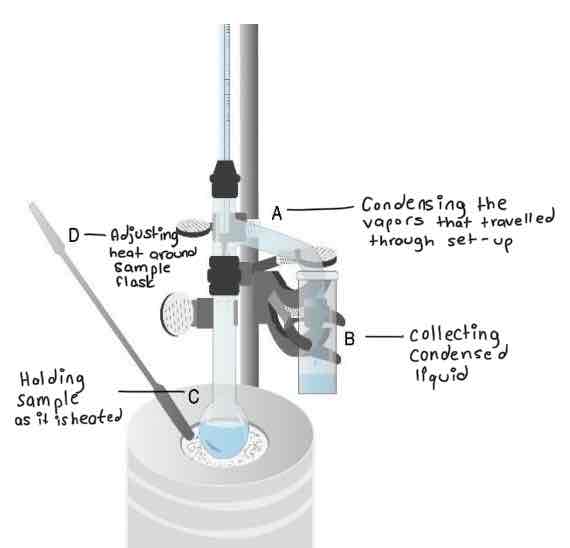
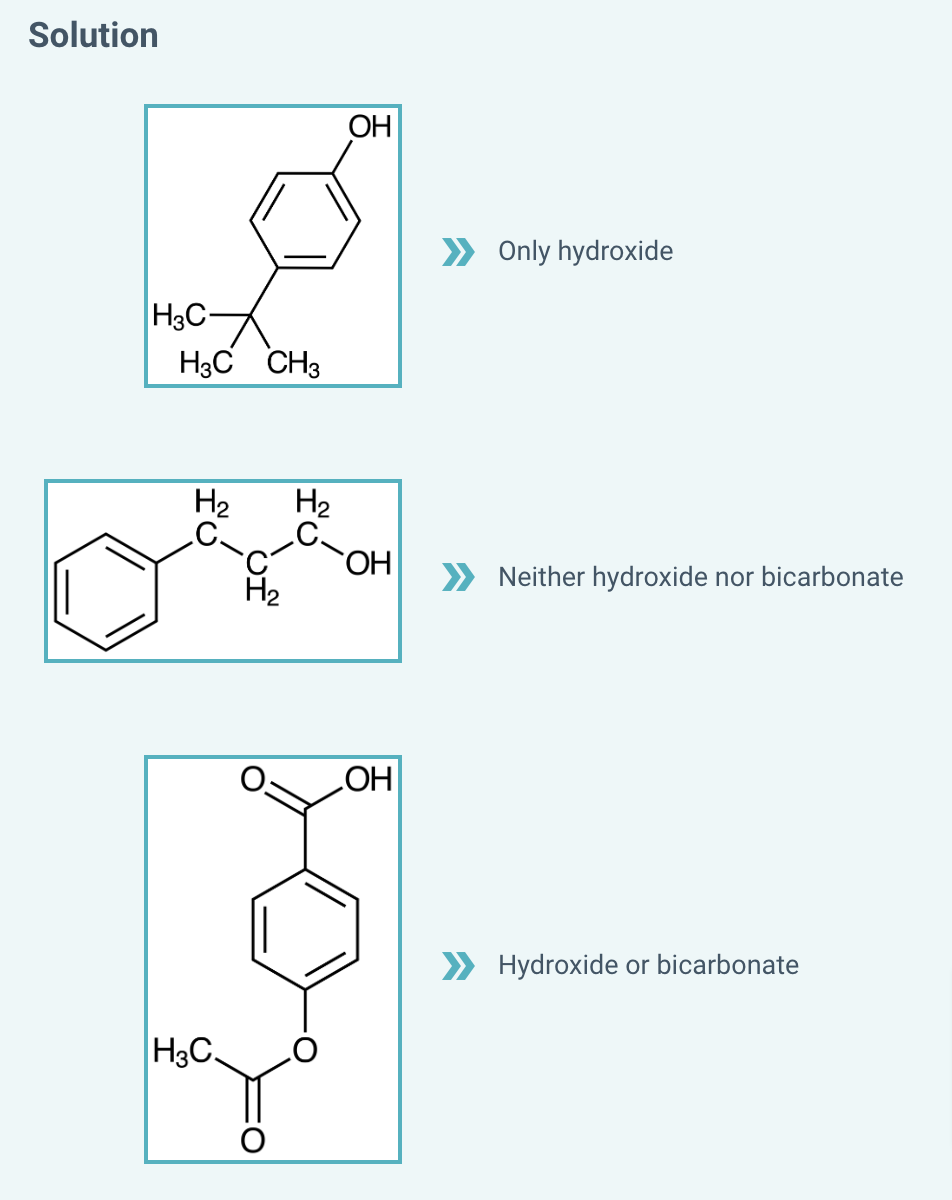
Determine which base will work to deprotonate each compound in an acid/base extraction
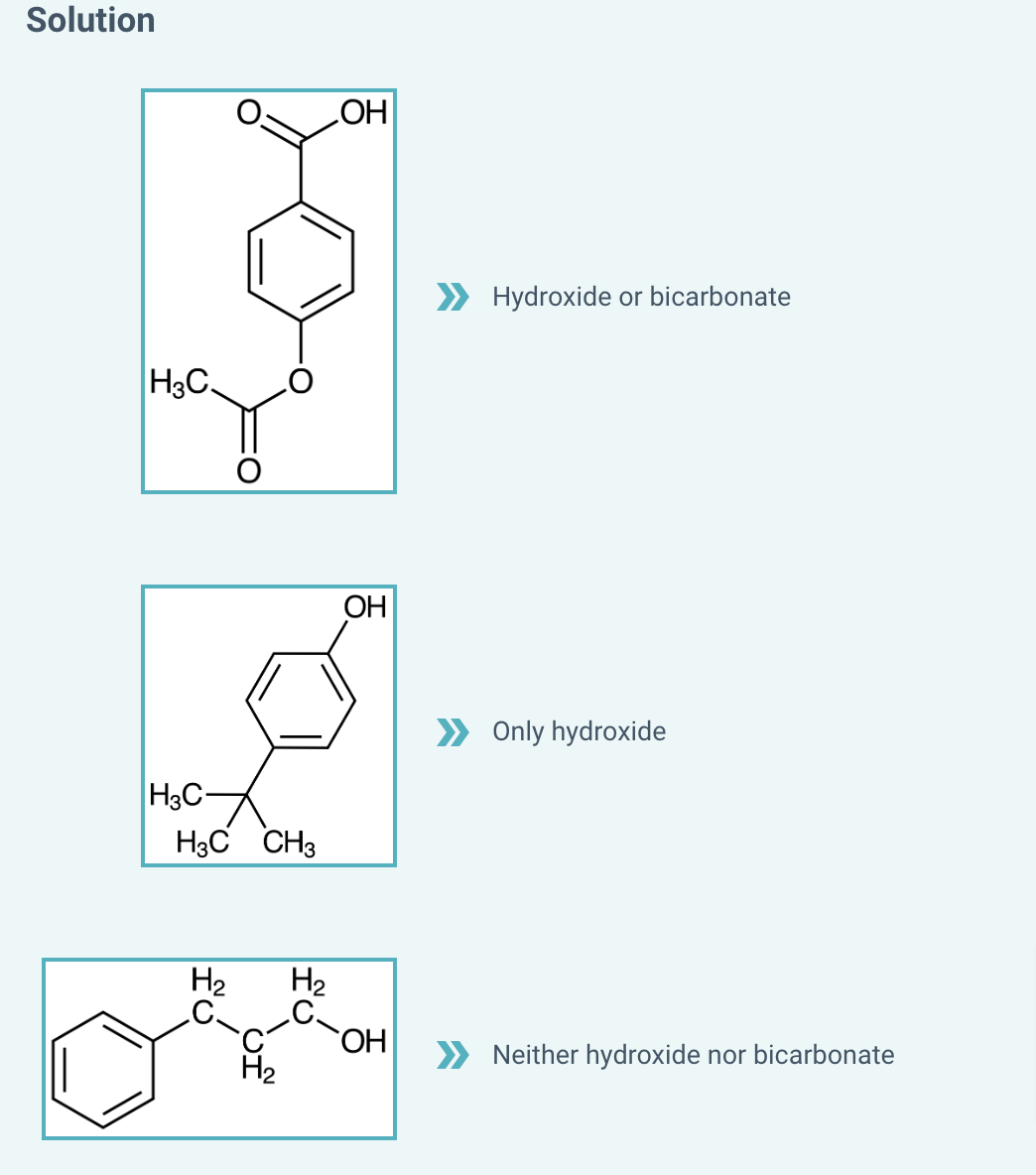
Washes and extractions are both techniques that use a separatory funnel to separate liquid layers. However, washes and extractions have differences.
Determine whether each statement applies to washes or extractions.
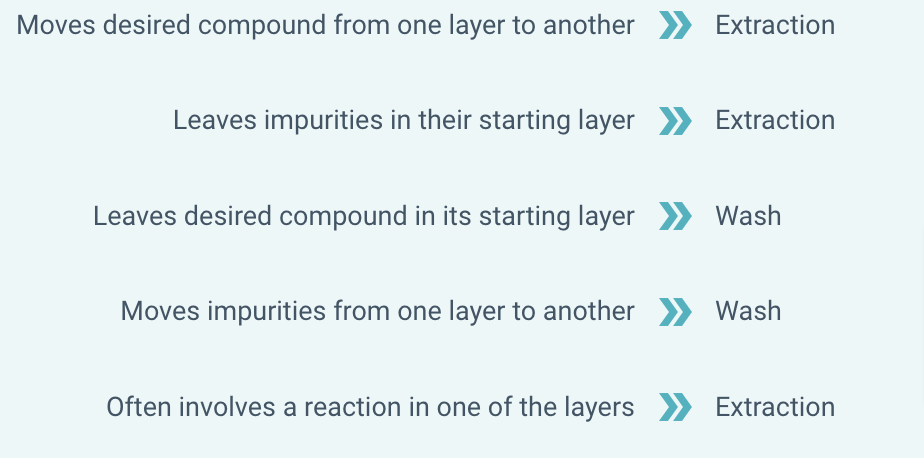
Suppose you are performing an extraction procedure with a carboxylic acid, like a benzoic or toluic acid.
Which layer should contain the carboxylic acid in a two-layer mixture of water and an organic solvent, like diethyl ether?
The organic layer
You then add a base to form the corresponding carboxylate.
Which layer should contain the carboxylate in a two-layer mixture of water and an organic solvent, like diethyl ether?
The aqueous layer
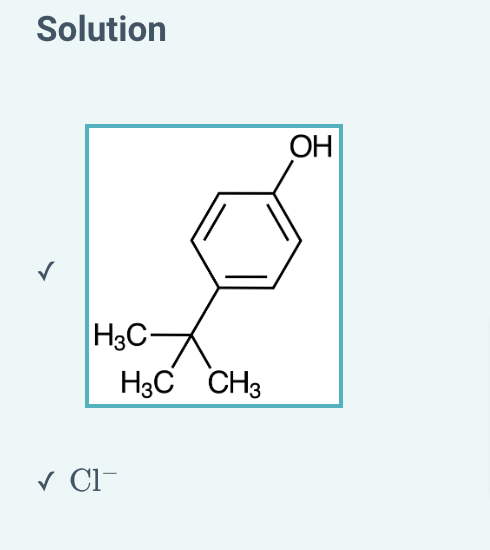
Suppose you are performing an acid/base extraction. After one of the steps, you have collected a
NaOH
extract containing the pictured phenolate.
Next, you add
HCl
to the layer. What are the products of the
HCl
addition?
Select one or more:
When using acids and bases, note that these substances are ____. Make every effort to avoid contact with ____ & ____. Be sure to wipe up any spills ____.
Corrosive - The Skin & Lab Surfaces - Immediately
Aniline involves an amine, which is a ____ functional group.
When an aqueous acid solution is added to an organic solution including aniline, the aniline appears in the ____ layer in its ____ form.
Then, a base is added to ____ the aniline.
Basic - Aqueous - Protonated - Reconstitute
When separating benzoic acid, naphthalene, and 3-nitroaniline, identify the solution used for each described purpose.

What hazards are associated with naphthalene?
Naphthalene is flammable.
Naphthalene is toxic.
Naphthalene produces nauseating vapors.
After mixing the solutions in a separatory funnel, the stopper should be ____ and the liquid should be ____ and the layers allowed to separate.
When you get close to the interface between the layers, ____ the funnel and ____ until the first layer is collected.
____ to collect the second layer.
Removed - Drained through the Stopcock - Get Eye Level with - Slow the Draining - Switch to a New Flask
When using a separatory funnel, you should burp the funnel.
What does the term burping mean?
Opening the stopcock when the flask is inverted during mixing
Determine which base will work to deprotonate each compound in an acid/base extraction.
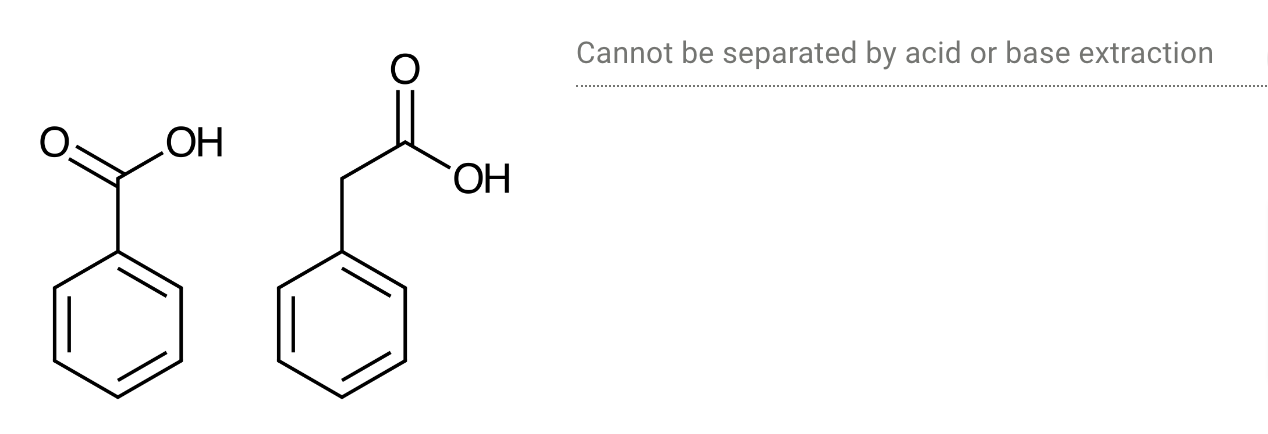
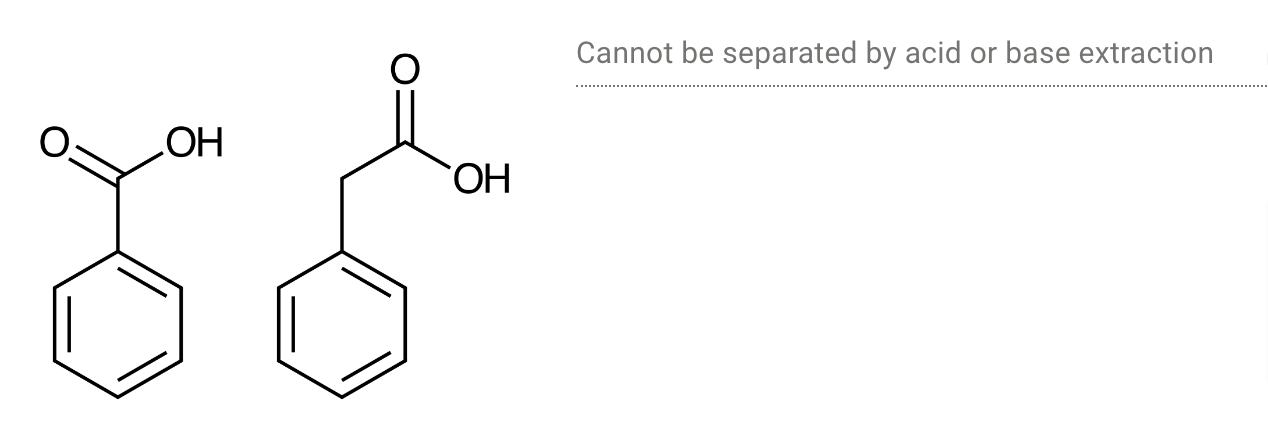
Determine whether each pair of chemicals could be separated by an acid or base extraction.
What is the purpose of a drying agent in the work up of an organic reaction?
To absorb small amounts of water in an organic solution
Identify the best practices when storing and using drying agents in the lab.
Close the drying agent container whenever it is not in active use.
Wrap the lid of the drying agent container with tape for storage.
What is the best course of action if solid material remains in the flask after the heating step of a recrystallization?
Filter the hot mixture.
After a recrystallization, a pure substance will ideally appear as a network of ____. If this is not the case, it may be worthwhile to reheat the flask and allow the contents to cool more ____.
Large Crystals - Slowly
In general, when hydrocarbons like oil are added to water, the two liquids ____ because hydrocarbons are ____ and water is ____.
Do Not Mix - Non-Polar - Polar
At the melting point, the solid form of a substance ____ the liquid form of the substance.
Is in Equilibrium W/
Determine whether each melting point observation corresponds to a pure sample of a single compound or to an impure sample with multiple compounds.

To prepare a sample in a capillary tube for a melting point determination, gently tap the tube into the sample with the ____ end of the tube down. Continue tapping until the sample ____.
Then, with the closed end of the tube down, tap the sample down slowly or ____ to move the sample down faster.
Finally, make sure that you can see ____ in the magnifier when placed in the melting point apparatus before turning on the heat.
Open - Is a Couple Millimeters High - Closed - Drop the Tube into a Longer Tube - Some or all the Solid
Why should you use a finely ground solid when determining the melting point of a sample?
Air pockets in a coarse sample could disrupt heat distribution.
Uniform, small particles heat more consistently throughout the sample.
What characteristics should a good sample for melting point determination have?
Solid phase
Thoroughly dry
Small particles
What is the recommended order of measurements to report the most accurate melting point possible?

When performing a melting point determination, how should you report your findings?
As a range from the temperature when melting starts to the temperature when it ends
When melting mixtures of compounds, what is the eutectic composition?
The mixture composition with the lowest melting point
Calculate the Rf value for a spot in a TLC experiment if the solvent moved 12.5 cm and the spot moved 8.2 cm from the origin.
To run a thin layer chromatography experiment with a chemical substance, begin by marking a horizontal line near the bottom of a TLC plate with ____. Place a ____ spot of the substance onto the line. For the mobile phase, add a small amount of ____ at the bottom of a TLC chamber. Place the plate in, then ____ the chamber. Once the mobile phase approaches the top of the plate, remove the plate and mark the line. Note the position of the spots and calculate Rf values if needed.
Pencil - Small - Solvent - Cover - Solvent
Identify the chromatography term that corresponds to each definition.

Identify the medical applications of aspirin.
Ant-inflammatory agent
Fever reducer
Pain killer
Identify the effect of each error during a thin layer chromatography experiment.

In a TLC experiment, how do you identify the components of an unknown mixture?
Compare the Rf values of the mixture components to the Rf values of pure known compounds.
Aspirin was formed to reduce the irritation of salicylic acid. However, aspirin can still disturb the stomach.
Carboxylic acid
Suppose you are going to perform a TLC experiment, which materials will you use to prepare the TLC development chamber?
Watch glass
Filter paper
Beaker
After performing a TLC experiment, you might place the TLC plate in a container with iodine crystals.
The crystals create a gas that reacts with organic compounds to help see them on the plate.
What is the best listed approach for viewing spots that are not visible on a TLC plate?
UV lamp
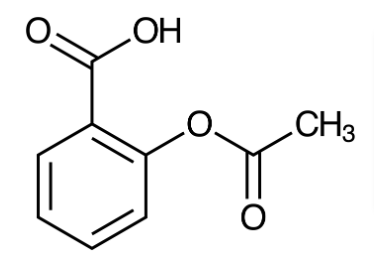
What is the correct structure of aspirin?