15.1-15.4 Quiz
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
Chemical equilibrium
the point at which the concentrations of all species are constant
Dynamic equilibrium
the point at which the rate od decomposition equals the rate of dimerization; opposing rates are equal

double arrow
implies process is dynamic

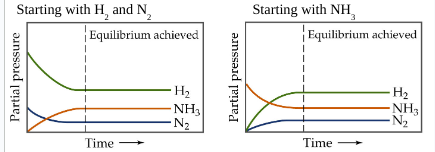
At equilibrium
[A] or kf[A] decreases to a constant, [B] or kf[B] increases from zero to a constant; [A] and [B] or kf[A] = kf[B] are constant
No matter the starting composition of reactants and products, the ______ ratio of concentrations is achieved at equilibrium
same
Equilibrium constant expression for a general reaction in the gas phase
Keq = (products)m/(reactants)n; Keqis equilibrium constant
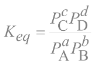
Law of Mass Action
expression of concentrations (partial pressures or molarities) of the reactants and products present at equilibrium
Equilibrium constant expression for everything in solution
Keq = [products]m/[reactants]n
![<p>K<sub>eq</sub> = [products]<sup>m</sup>/[reactants]<sup>n</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/bd8e3f5d-d8ea-4da0-9d75-9a8a97fd129a.png)
Keq
based on molarities of reactants nad products at equilibrium; omit units; same equilibrium established no matter the beginning; products/reactions; equilibrium constant
Keq » 1
products dominate at equilibrium and the equilibrium lies ot the right
Keq « 1
reactants dominate at equilibrium and the equilibrium lies ot the left
Equilibrium can be approached from ____ direction
any
Keq reverse reaction
1/Keq
When the stoichiometric coefficients of a reaction are multiplied by a factor c, K is
raised ot the power c
When reactions are added together, the K of the resulting overall reaction is
the product of the K’s for the reactinos that were summed
How are equilibrium constants for gas phase reactions expressed?
expressed partial pressures not concentrations since pressure is proportional to the concentration; P =MRT & M = P/RT
Homogeneous
all reactants and products are in one phase
Heterogeneous
one or more reactants or products are in a different phase
Concentrations of solids and pure liquids are
constant so ignore them in Keq