Chemistry-Test For Cations & Anions
0.0(0)
Card Sorting
1/9
Earn XP
Last updated 6:19 PM on 12/18/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards

Anions
Negatively Charged ions - Can be tested As:
Carbonate
Halides
Nitrate
Sulphate
Sulfite
2
New cards
Carbonates Test
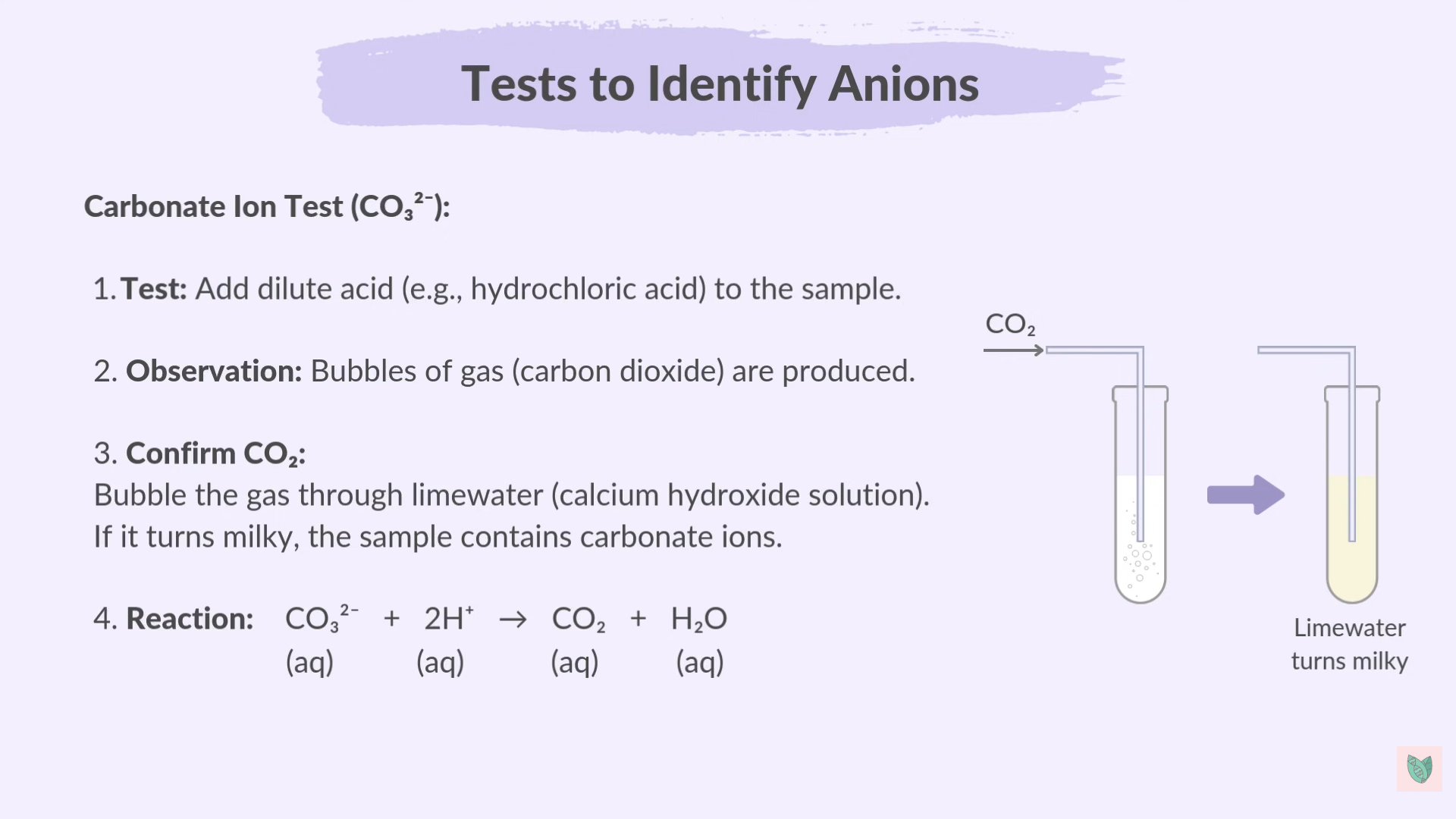
3
New cards
Halides Test

4
New cards
Nitrates Test
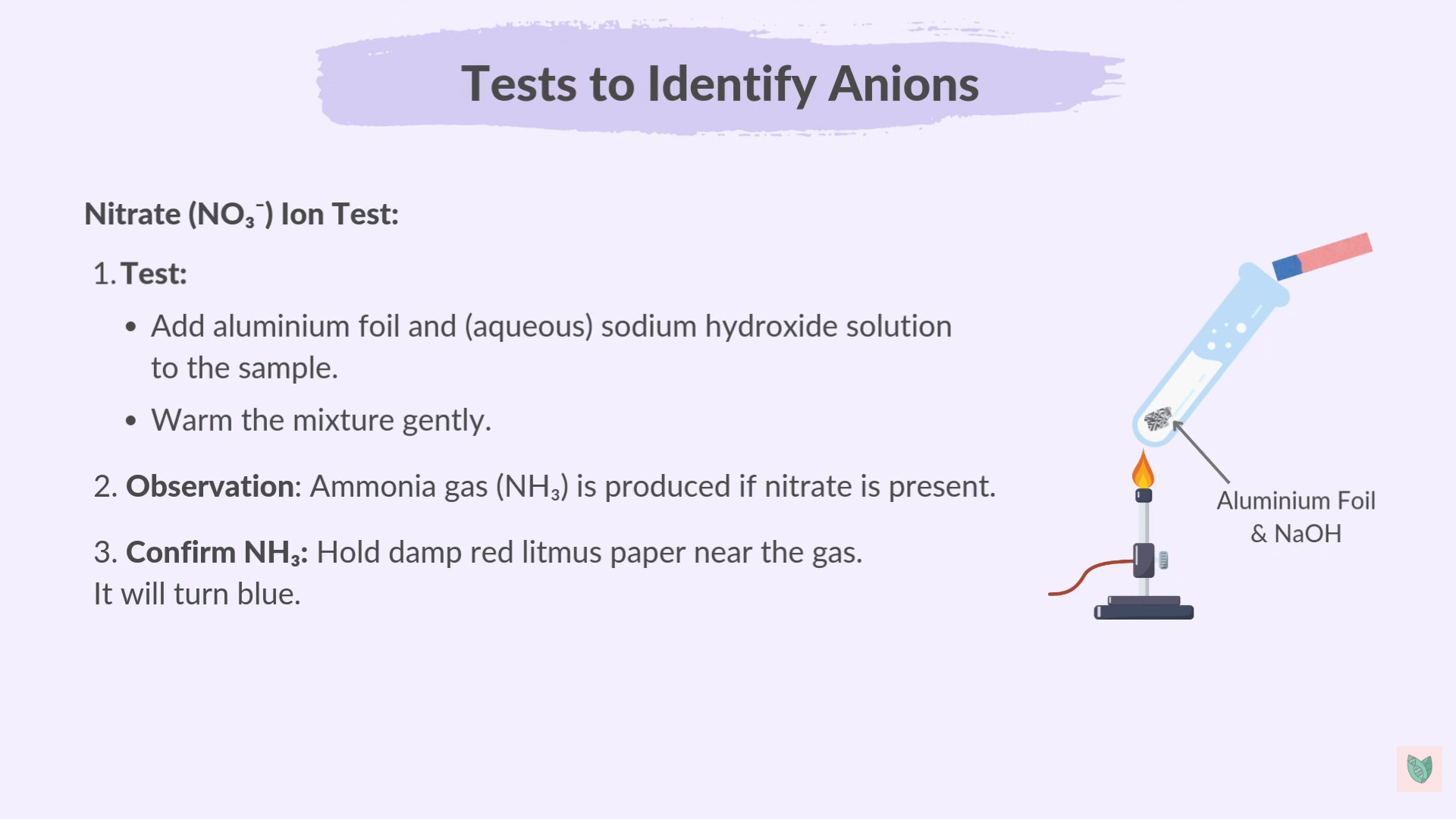
5
New cards
Sulfate Test

6
New cards
Sulfite Test
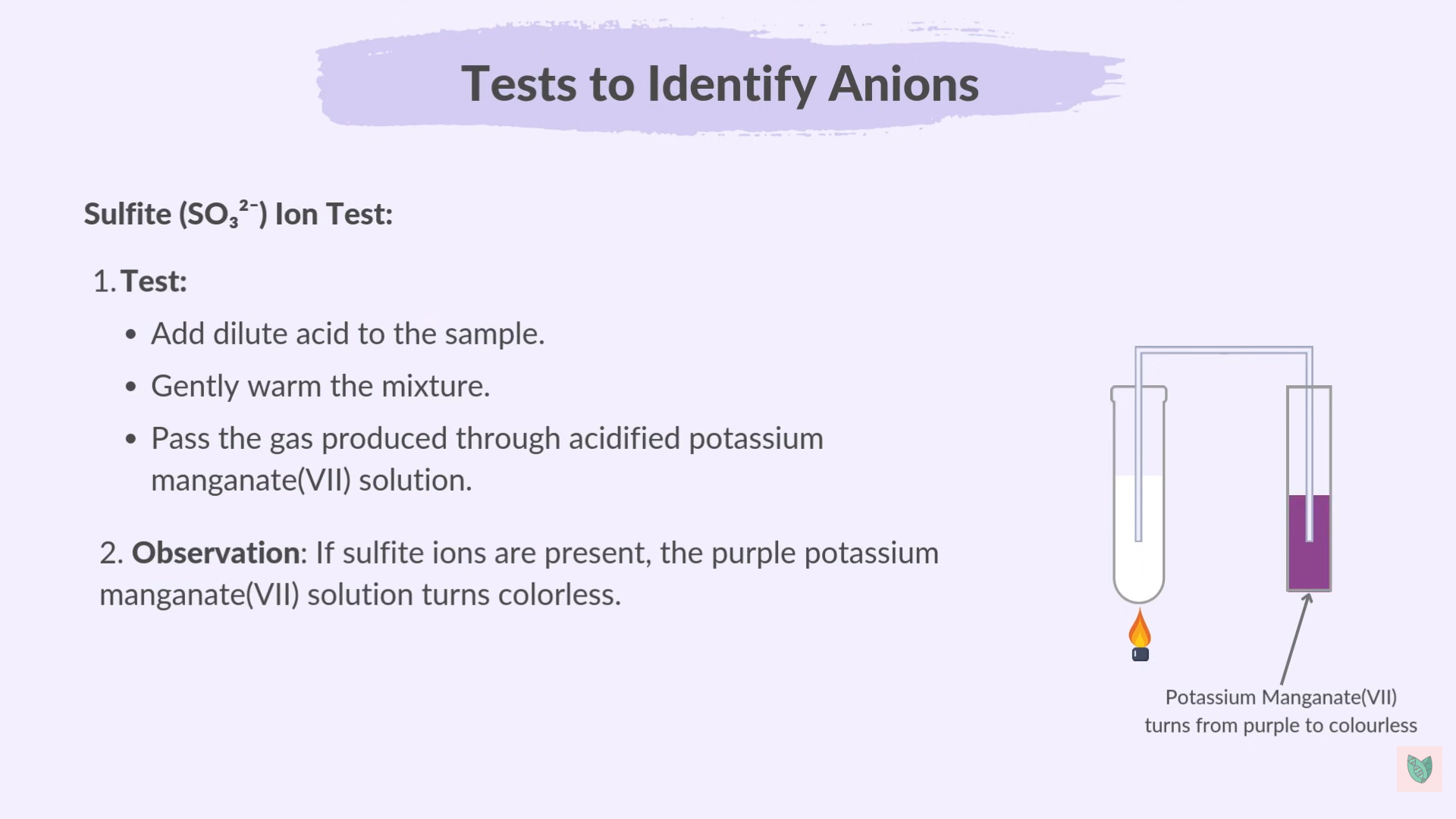
7
New cards
Cations
Positively Charged ions - Include:
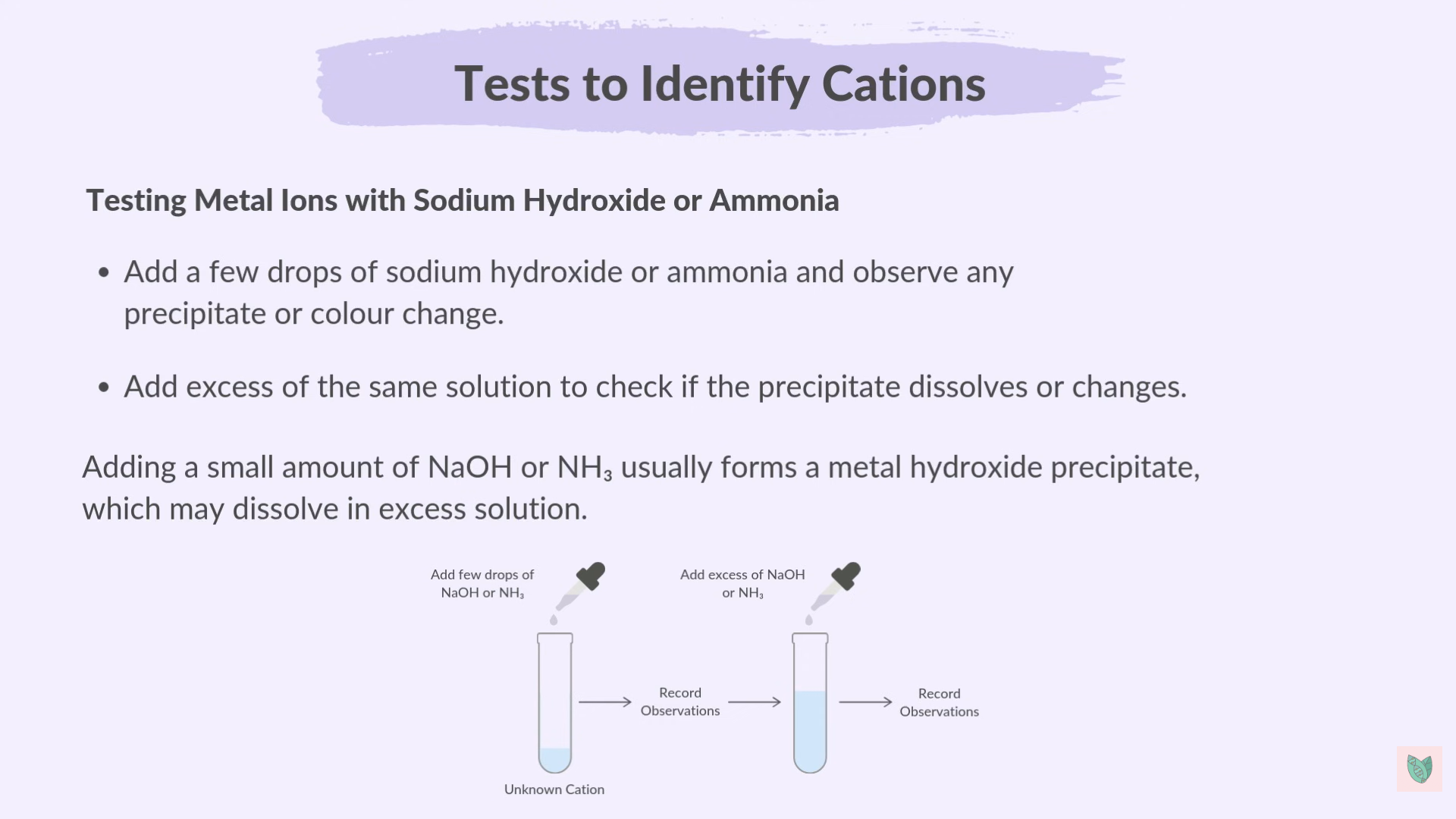
Ammonium Ions
Metal Ions with sodium Hydroxide or Ammonia

8
New cards
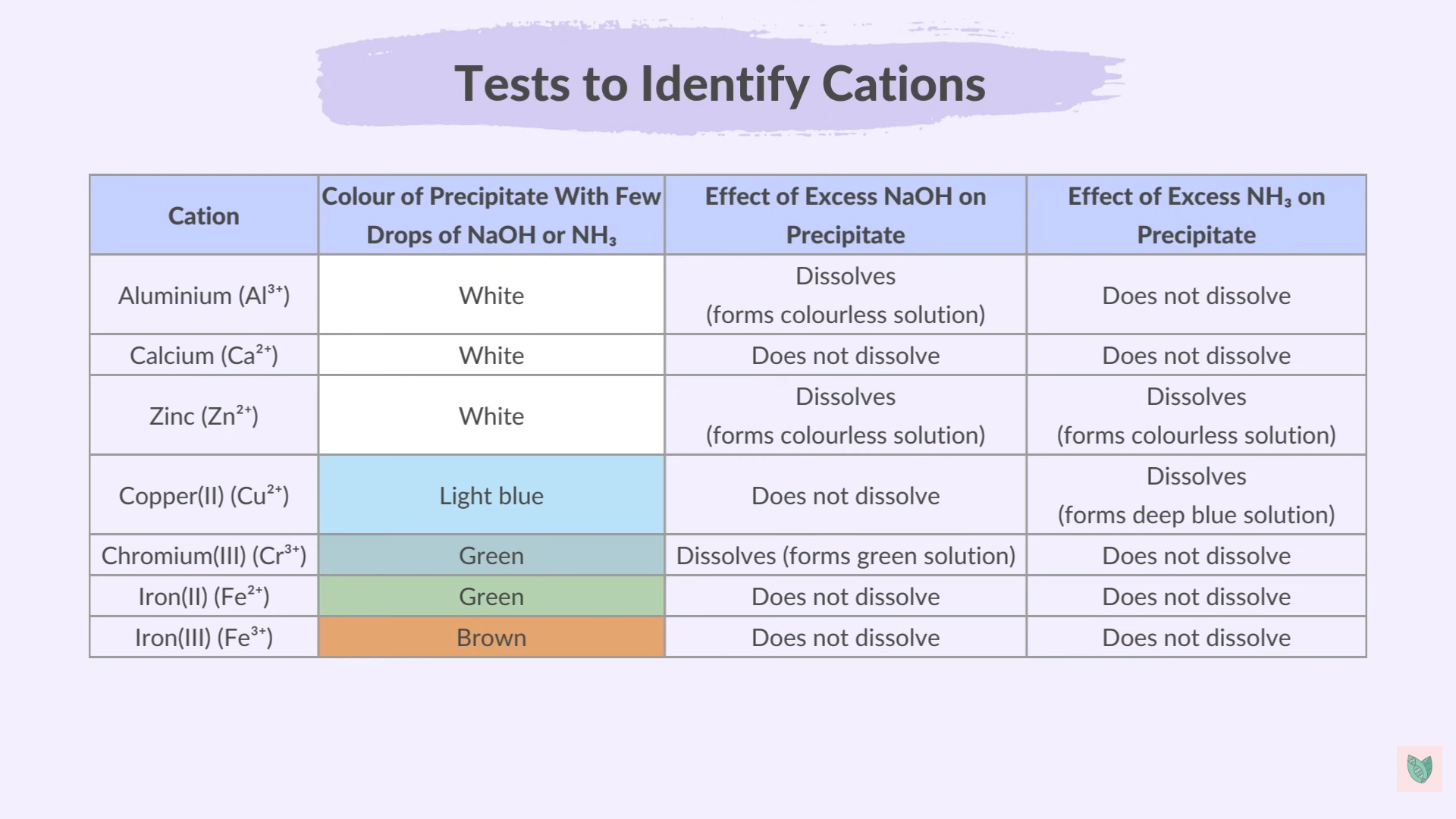
Other Cations
Include
Aluminum
Calcium
Zinc
Copper
Chromium (III)
Fe (II)
Fe (III)
9
New cards
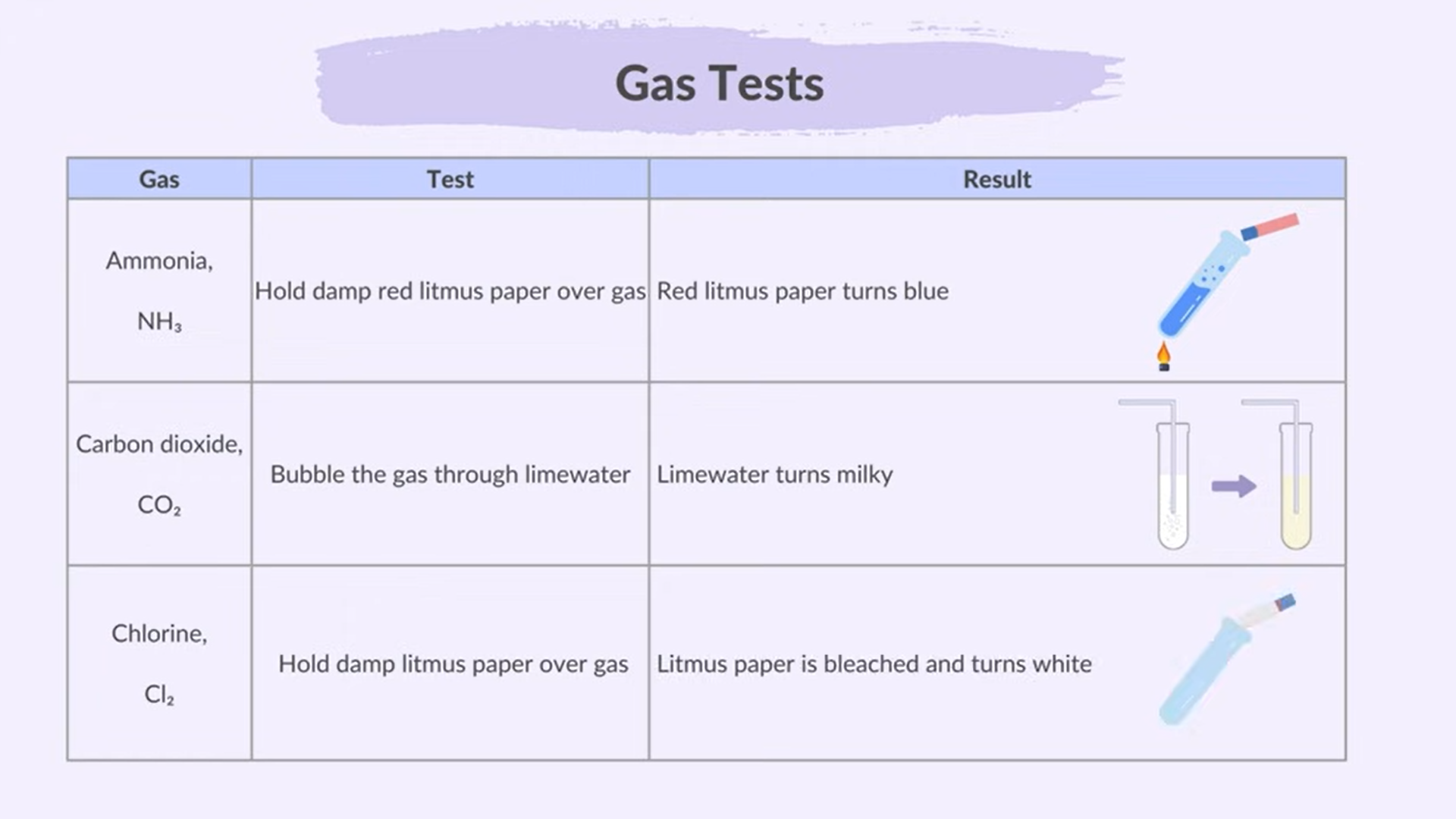
Test for Gases
Hydrogen
Oxygen
Sulfur
Ammonia
Carbon dioxide
Chlorine

10
New cards

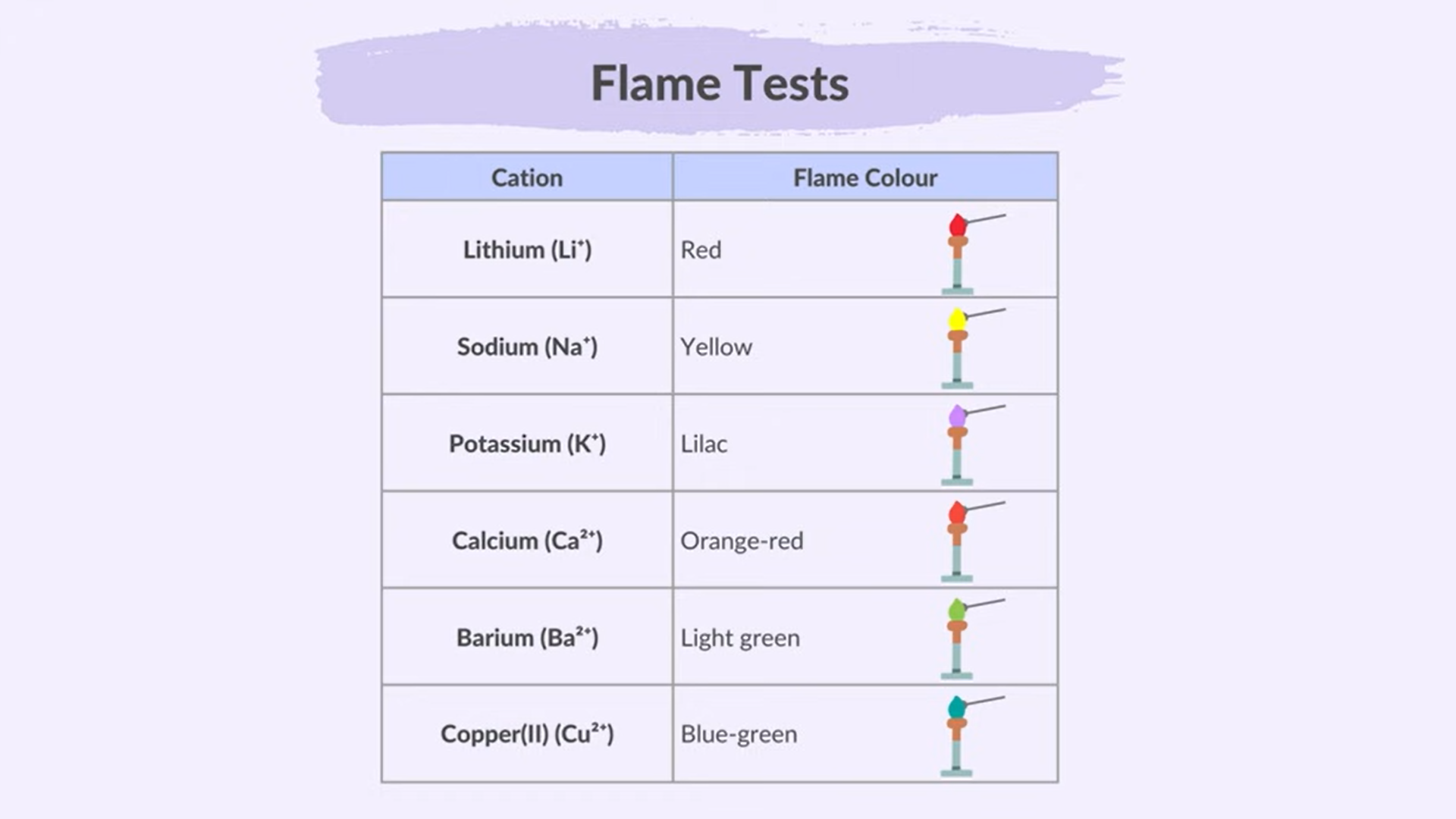
Flame Test For Metal ions
Steps:
use a loop made of unreactive metal (platinum)
Clean by dipping it in HCl And heating Until no Color appears
Then Dip it into the sample
Heat and Observe
Colors:
BLC-PS-C
Barium: Light Green
Lithium: Red
Calcium: Orange-Red
Potassium: Lilac
Copper: Blue
Explore top notes
Explore top flashcards
CRISC - Certified in Risk and Information Systems Control term definition - Part 53
20Updated 1207d ago0.0(0)
CRISC - Certified in Risk and Information Systems Control term definition - Part 53
20Updated 1207d ago0.0(0)