Chapter 5: Thermochemistry
Energy
Energy is the ability to do work or transfer heat (SI Unit: Joule)
Thermodynamics is the study of energy and its transformations.
Thermochemistry is the study of chemical reactions and the energy changes that involve heat.
Potential energy - stored energy
Kinetic energy - energy matter in motion
Chemical energy is a form of potential energy.
First Law of Thermodynamics
Energy is never created or destroyed, but it can be converted from one form to another.
Chemical energy can be converted to heat to heat homes.
Sunlight is converted to chemical energy in green plants.
Systems and Surroundings
The portion of the universe that we single out to study is called the system.
The surroundings are everything else.
Example 1:
Molecules in a piston - the system.
The piston - the surroundings
Example 2:
Ice - the system
Everything else - the surrounding
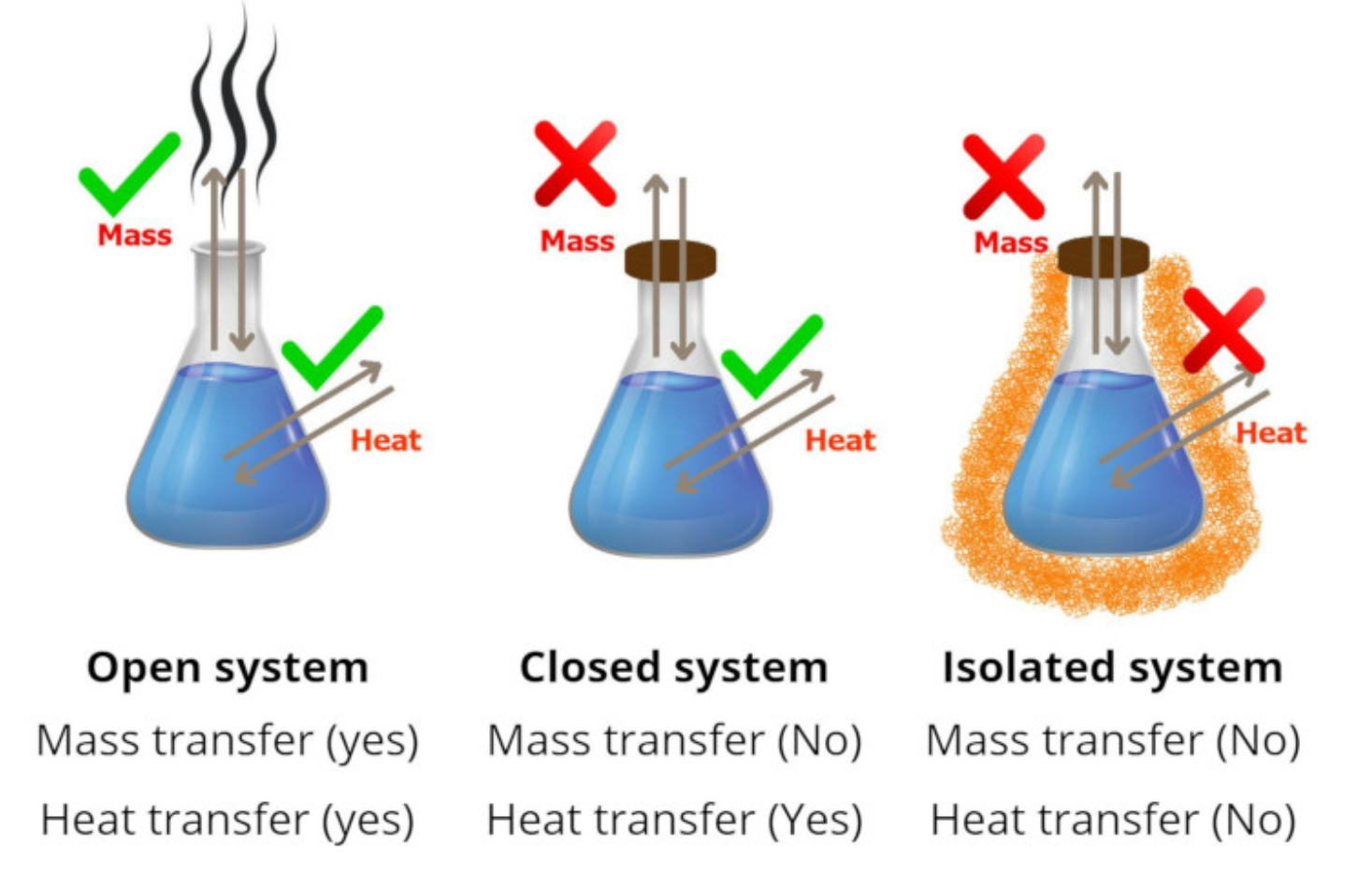
Types of Systems
Open System: a region of the universe being studied that can exchange heat and mass with its surroundings.
Closed System: a region of the universe being studied that can only exchange heat with its surroundings (not mass).
Isolated System: a region of the universe that can not exchange heat or mass with its surroundings.
Internal Energy
The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we use E to represent it.
We don’t generally know E, only how it changes.
The change in internal energy is \Delta E , the final energy of the system minus the initial energy of the system.
Equation for change in energy: \Delta E=E_{final}-E_{initial}
Or \Delta E=E_{products}-E_{reactants}
Changes in Internal Energy
If \Delta E>0, then E_{final} >E_{initial}
The system absorbed energy from the surroundings (endothermic)
If \Delta E<0, then E_{final} <E_{initial}
The system released energy to the surroundings, which is an (exothermic)
When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w).
Heat (q)
Work (w)
Equation for changes in internal energy: \Delta E=q+w
The systems always results in an increase in the internal energy of a system when it gains heat and has work done on it by the surroundings
Thermodynamic Quantities
Thermodynamic quantities have three parts
A number
A unit
A sign
Note about the sign:
A positive (+) \Delta E results when the system gains energy from the surroundings.
Endothermic
A negative (-) \Delta E results when the system loses energy to the surroundings.
Exothermic
Change is energy, heat, work, and their signs
Heat, Work, and Energy | Positive | Negative |
For q (heat) | + means system gains heat | - means system loses heat |
For w (work) | + means work done ON system | - means work done BY system |
For \Delta E (energy) | + means net gain of energy (heat and work) by system | - means net loss of energy (heat and work) by system |
Exchange of Heat Between System and Surroundings
When heat is absorbed by the system from the surroundings, the process is endothermic.
When heat is released by the system into the surroundings, the process is exothermic.
State Functions
We know that the internal energy of a system is independent of the path by which the system achieved that state.
Internal energy is a state function.
It depends only on the present state of the system, not on the path by which the system arrived at that state.
\Delta E depends only on E initial and E final.
E is a state function
q and w are not state functions
Work
Usually the only work done by chemical or physical change is the mechanical work associated with a change in volume of gas.
We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston: w=-P\Delta V
The work is negative because it is work done by the system.
Work = force x distance
Enthalpy
Enthalpy (H) is the internal energy plus the product of pressure and volume (the sum of the energy change from a chemical reaction and the total work during the chemical reaction).
H=E+PV
When the system changes at constant pressure, the change in enthalpy \Delta H,
\Delta H=\Delta E+P\Delta V
A process is endothermic (absorbs energy) when \Delta H is positive.
A process is exothermic (released energy) when \Delta H is negative.
Enthalpy of Reaction
This quantity, \Delta Hrxn , is called the enthalpy of reaction (or heat of reaction).
The Truth About Enthalpy
Enthalpy is an extensive property, meaning it does depend on the amount of material present.
The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to \Delta H for the reverse reaction.
A + B → C is \Delta H=+
C → A + B is \Delta H=-
The enthalpy change for a reaction depends on the states of the reactants and the products (s,l, g, aq).
Calorimetry
Calorimetry: the measurement of heat flow.
Since we cannot know the exact enthalpy of the reactants and products, we measure \Delta H through calorimetry.
Calorimeter: instrument used to measure heat flow.
Calculating the amount of heat
q=mc\Delta T
q is heat
m is mass
c is the constant (specific heat)
Common constant is the specific heat of water (4.184\:J/g\cdot C)
\Delta T is change is temperature (final T - initial T)
Heat change between surroundings and the system
q_{soln }=-q_{rxn}
Hess’s Law
We can calculate \Delta H using published \Delta H values and the properties of enthalpy.
Hess’s law: If a reaction is carried out in a series of steps, \Delta H for the overall reaction equals the sum of the enthalpy changes for the individual steps.
\Delta H is a state function, so for a particular set of reactants and products, \Delta H is the same whether the reaction takes place in one step or in a series of steps.
Enthalpies of Formation
Enthalpy of formation (\Delta H_{f}): the enthalpy change for the reaction in which a compound is made from its constitute elements in their elemental forms.
Calculation of \Delta H
We can use Hess’s law in this way: \Delta H=\sum n\Delta H_{f,products}-\sum m\Delta H_{f,reactan ts}^{°}, where n and m are the stoichiometric coefficients.
Bond Enthalpy
The bond enthalpy is always positive because energy is required to break chemical bonds.
Breaking bonds requires energy.
Forming bonds releases energy.
The greater the enthalpy, the stronger the bond.
Bond Enthalpies and Enthalpy of Reaction
Add bond energy for all bonds made (+)
Subtract bond energy for all bonds broken (-)
We can predict whether a chemical reaction will be endothermic or exothermic using bond energies.
\Delta H_{rxn}=\sum bonds\:broken-\sum bonds\:formed
Reference: Chemistry The Central Science (14th Edition)