Alkene reactivity and as electophiles
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
A double bond with a region of high electron density is often refered to as?
Isolated so double bonds are nucleophilic
Why do we get regioisomers?
Because of it’s carbocation intermediate being the most stable e.g secondary > primary
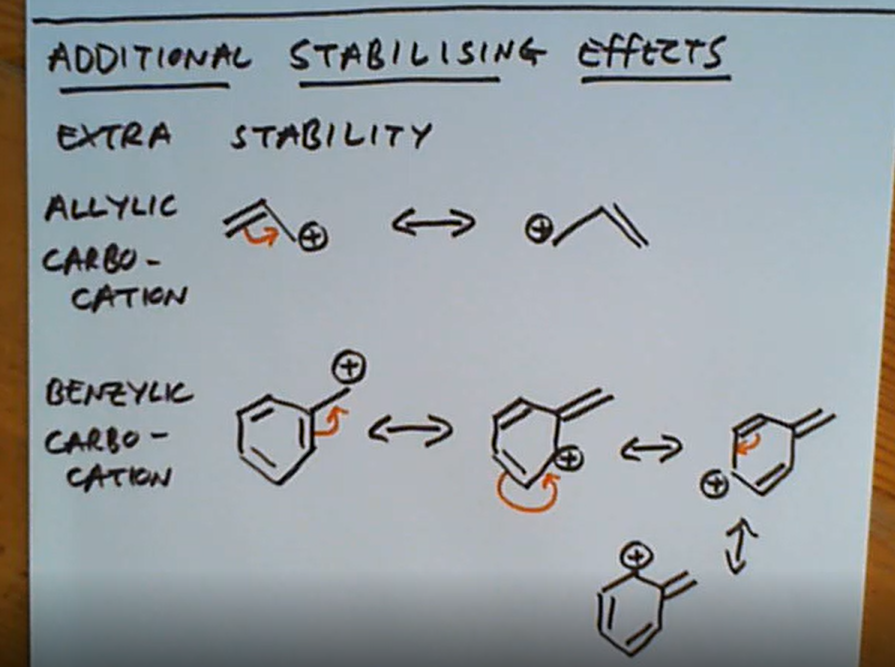
What 2 other resonance structures provide extra stability?
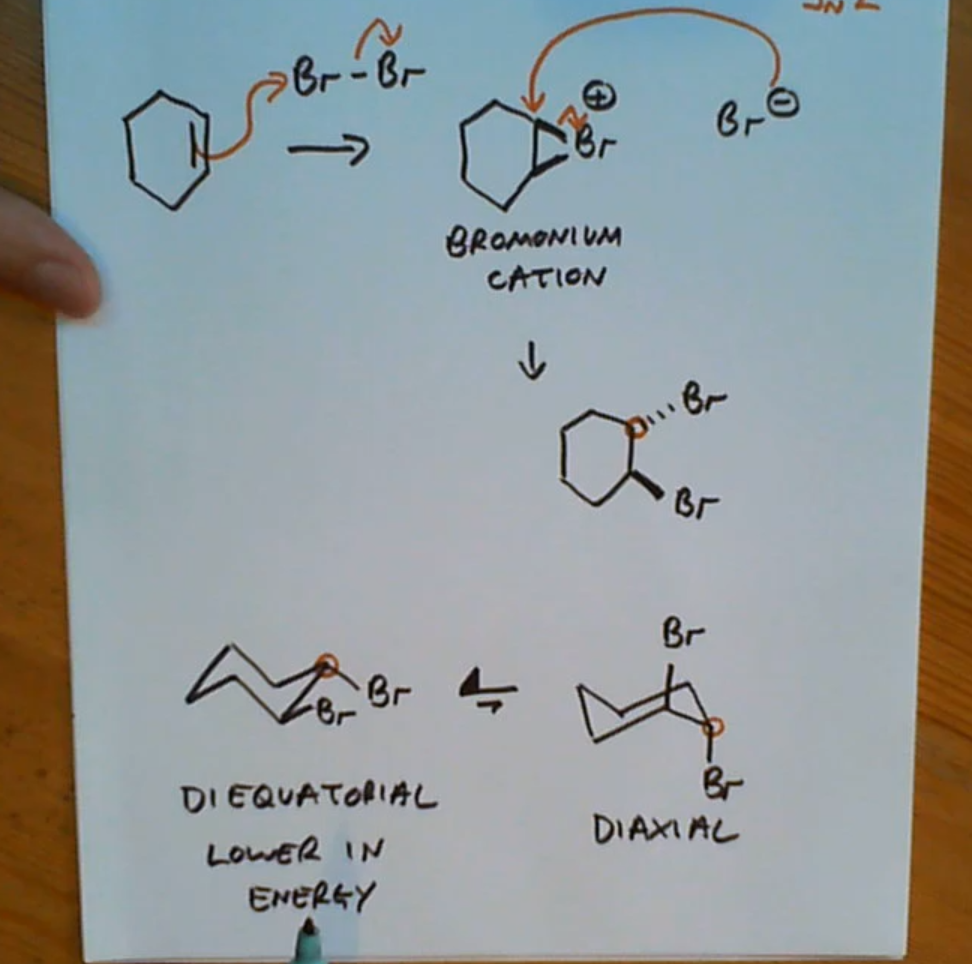
Phenyl with a double bond reacting with Br2 and the chair conformation product
Can aromatics just react with nucleophiles?
NO! we need a lewis acid because they are poor nucleophiles
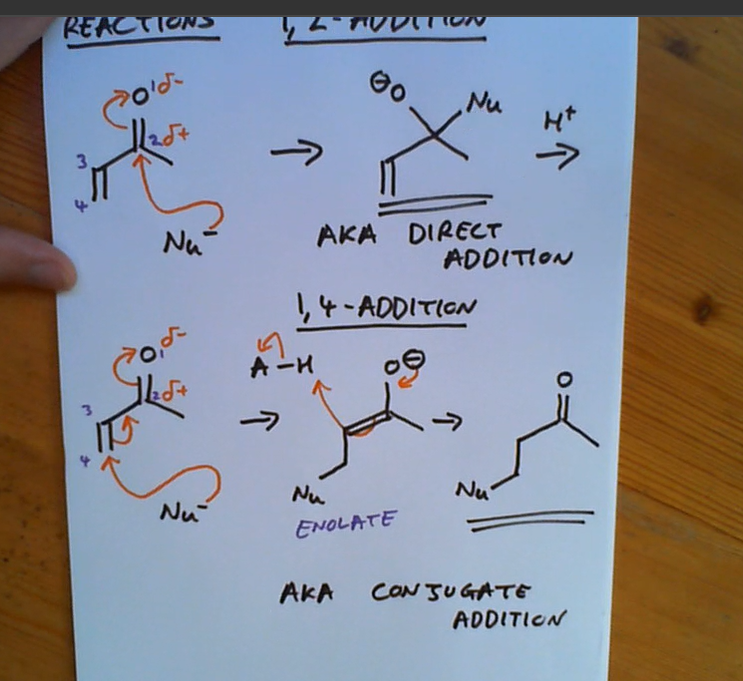
What special about nucleophiles attacking an enone?
Can attack from 2 different positions
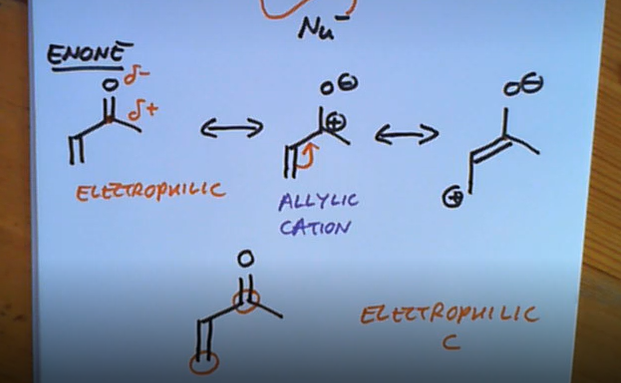
An enone’s reaction with a nucleophile
aka michael additon for 2nd
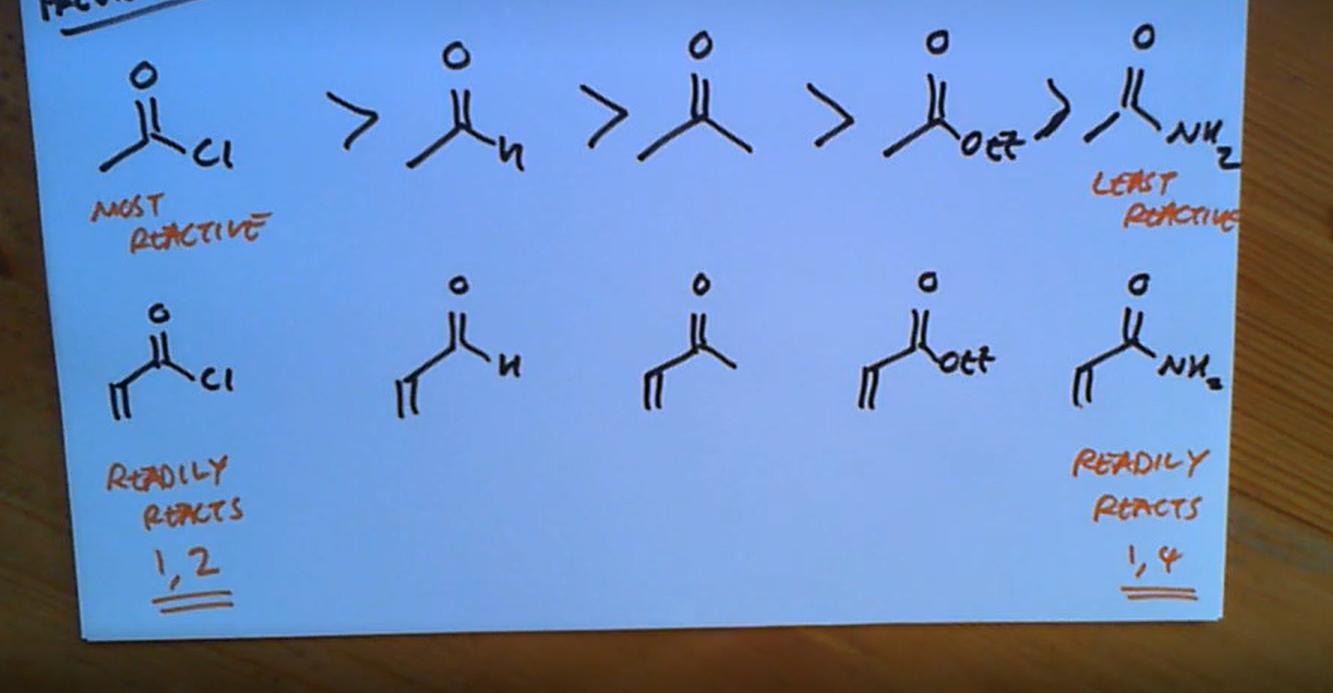
Order of reactivity in alpha beta unsaturated form, from order of readily reacts 1,2 to 1,4
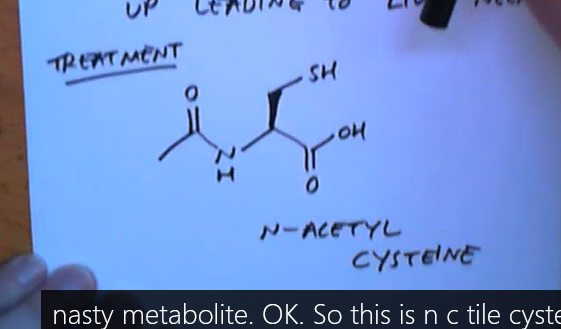
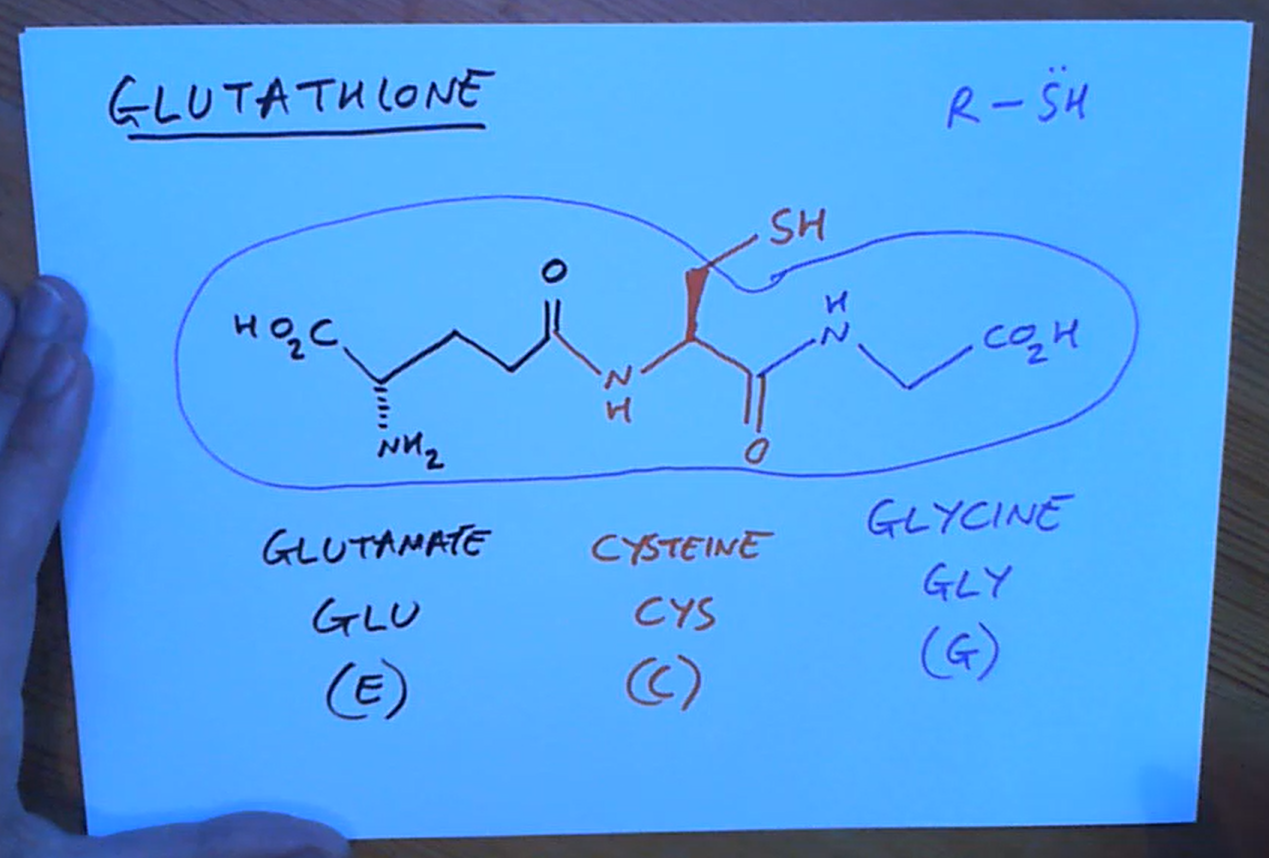
What is glutathione used for?
To detoxify compounds in our body as it sacrifices itself reacting with them as a R-SH protecting our enzymes , etc. SH is a very good nulceophile
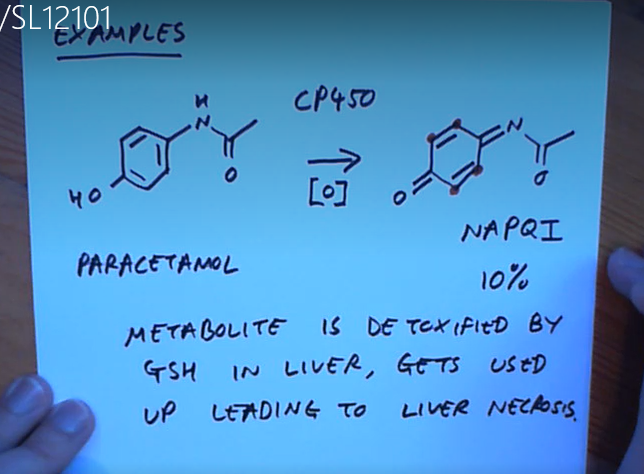
Why do we have limits on how many paracetomol to sell?
Because eventually we run out of glutathione and NAPQI starts reacting with enzymes in our liver leading to liver failure!
What is given to patients who overdose on paracetamol?