Catalysts
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
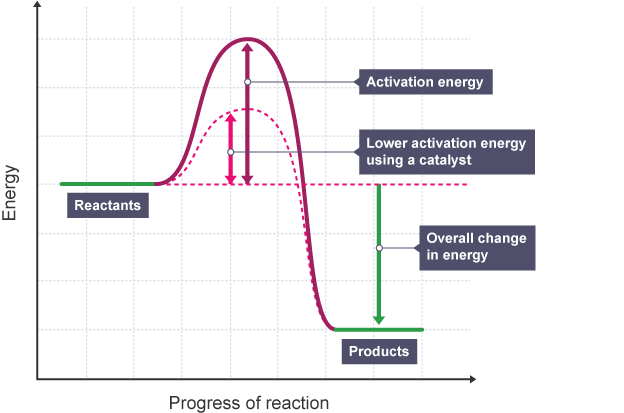
What is a catalyst?
A catalyst increases the rate of reaction by providing an alternative reaction pathway with a lower activation energy without being used up.
Many catalysts would usually only work on a single reaction.
How do catalysts work?
The catalyst lowers the activation energy, meaning there are more particles with enough energy to react when they collide.
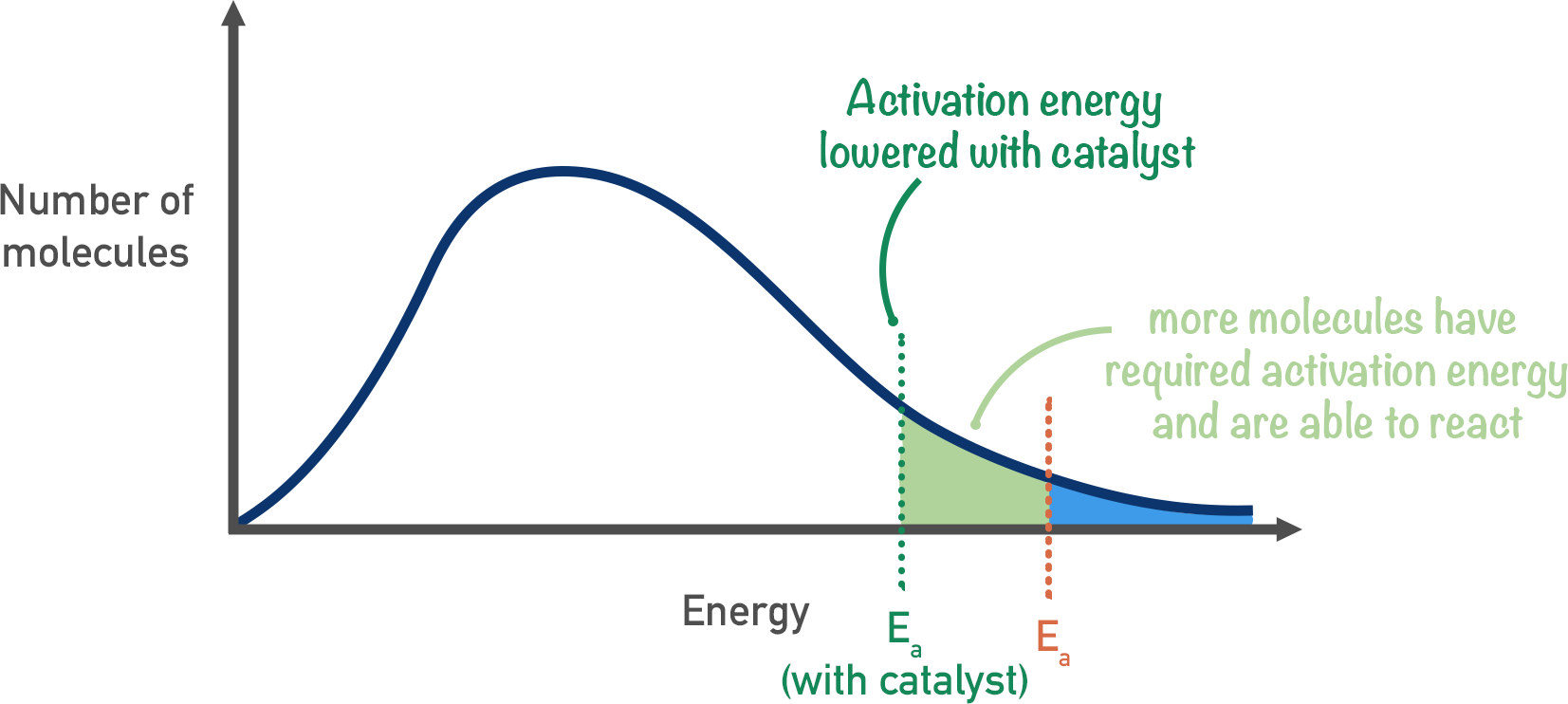
What does the Maxwell-Boltzmann distribution look like when a catalyst is added?
With a catalyst present, the molecules still have the same amount of energy, so the curve itself is unchanged.
But because the activation energy is now lower, more molecules have energies above this threshold and are able to react
hello
hello