General organic chemistry
1/19
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
What is the series that we get for basicity when the amine group, which is attached to methyl, reacts with water?
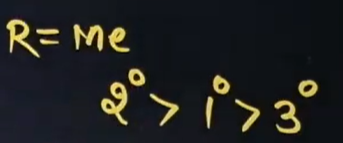
What is the series that we get for basicity when the amine group, which is attached to ethyl, reacts with water?
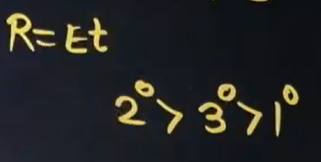
What is back bonding?
Back bonding is a process that creates a pi bond
What is a localised lone pair in resonance?
Lone pair electrons that do not take part in resonance
What is a un-localised lone pair in resonance?
Lone pair electrons that do take part in resonance
Which is the position on which M effect doesent work on in a cyclo compound?
Meta position on the cyclo compound
What is an activating and deactivating group?
Activating group → A group that causes electron density to increase in a benzene ring
Deactivating group → A group that causes electron density to decrease in a benzene ring
What type of directors are +M and -M groups?
+M → ortho and par directory
-M → Meta directory
What is the definition of hyperconjugation?
It involves the delocalisation of sigma electrons in C—H (C sp3—H s) of an alkyl group directly attached to an atom of an unsaturated system (alkenes, alkynes) or an atom of an unshared p orbital
Why do CO and CN not show hyperconjugation?
It is because they have a very high energy pi antibonding orbital present in them
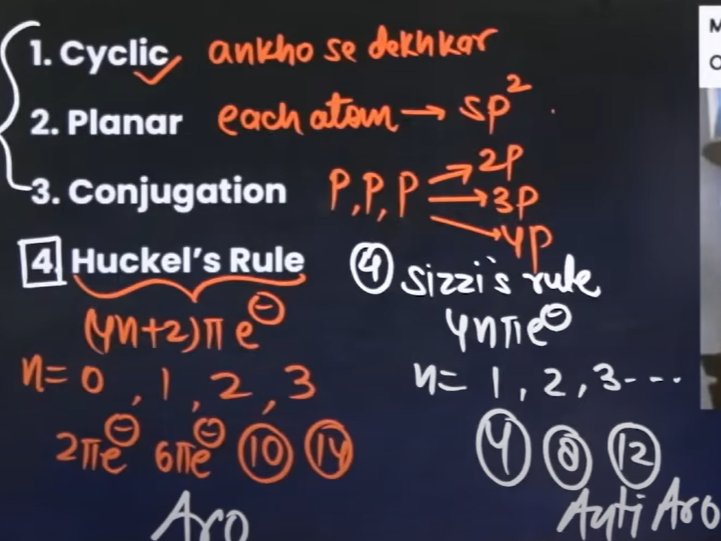
What are all the rules to check the aromaticity of compounds?
n → Number of electrons in a pi bond
If it’s in multiples of 4, then it isn’t aromatic
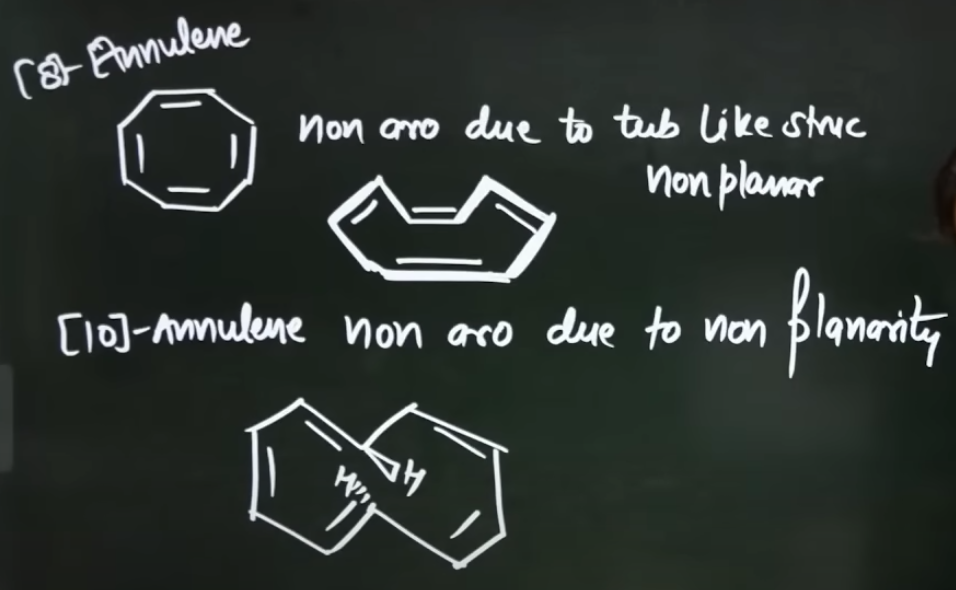
What are the 2 compounds that show aromaticity due to them not being planar but still technically satisfy all the 3 conditinos for a compound to be aromatic?
What is the definition for resonance energy?
What is the order of acidic strengths in different families?
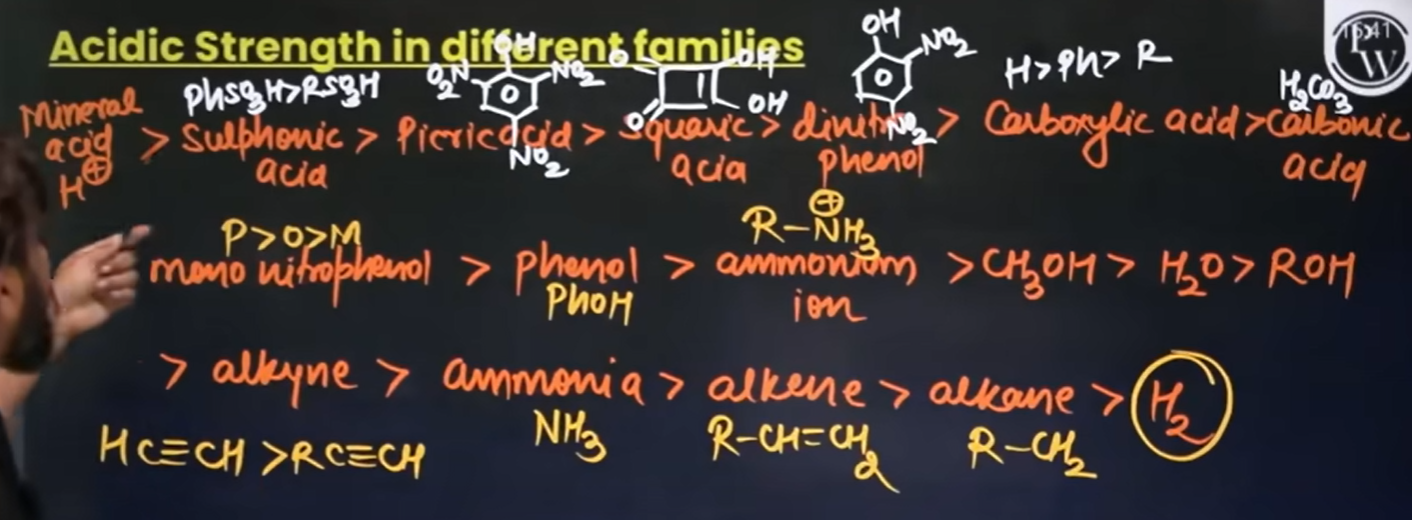
What is the acidic compound that shows the same acidic value as 7.1, which is similar to H2CO3? Show its structure
Para nitrophenol
What is tautomerism?
It is the intramolecular proton transfer (Proton transfer inside the same molecule; in itself)

What are the structures of the following compounds?
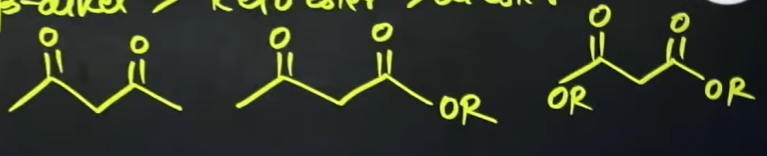
What are electrophiles?
Compounds have or do not have vacant orbitals (Have a charge on them or are neutral), thus are electron-loving species
What are nucleophiles?
Compounds have or do not have excess electrons (Have a charge on them or are neutral). The charge is due to a deficiency of electrons or due to pi electrons being present; they are electron-hating species
What are ambident nucleophiles?
It is a nucleophile that has two different atoms (or sites) that can donate electrons to attack an electrophile, but can only donate from one side at a time