Oxidative Phosphorylation
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
Malate- Aspartate Shuttle is apart of
Aerobic Metabolism
Reasoning for Malate-Aspartate Shuttle
NADH from glycolysis in the cytosol needs to get to the matrix for Oxidative Phosphorylation. However, NADH cannot move across the inner mitochondrial membrane and instead must use a shuttle system.
NADH used in the Malate-Aspartate Shuttle comes from
Glycolysis
NADH from glycolysis is recycled by ___
Cytosolic Malate DH
Malate-Aspartate Shuttle Linkage
uses malate, OAA, and Asp (which are present in both the cytosol and the membrane)
OAA + NADH ⇌ Malate + NAD⁺ (Malate DH)
OAA + NH₃ ⇌ Aspartate
OAA cannot go through the matrix membrane, but aspartate can
Malate is used as a __ by the Malate-Aspartate Shuttle
H⁺ carrier
The Malate-Aspartate Shuttle recycles
Glycolysis NADH
Chemiosmotic Theory
An H⁺ gradient drives ATP Synthesis
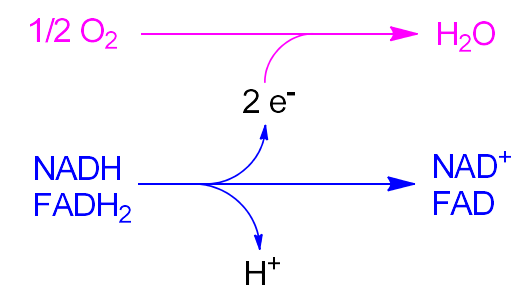
The H⁺ gradient is made by an oxidative process that reduces O₂ to H₂O
The Oxidative in Oxidative Phosphorylation refers to
Electron Transport Chain (ETC)
removes 2e⁻ from NADH, FADH₂, and adds them to O₂
makes a H⁺ gradient that drives ATP Synthesis
__ drives ATP Synthesis
H⁺ gradient
The Oxidative in Oxidative Phosphorylation includes the following equations
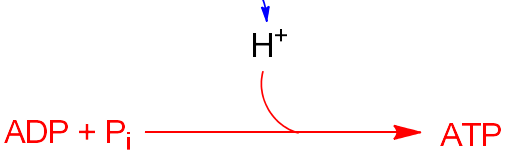
The Phosphorylation in Oxidative Phosphorylation includes the following equations
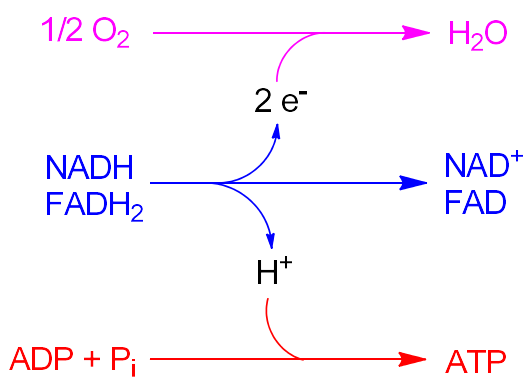
Oxidative Phosphorylation Formulas
The ____ part of Oxidative Phosphorylation makes an H⁺ gradient
Oxidative
The ___ part of Oxidative Phosphorylation uses an H⁺ gradient
Phosphorylation
The Phosphorylation part of Oxidative Phosphorylation
ATP Synthesis
uses H⁺ gradient to make ATP
produces most of ATP made in aerobic cells
Mitochondria
Electron Transport Chain
ATP Synthase (Ox Phos)
Mitochondria Inner Membrane Space
H⁺ gradient (Ox Phos)
Matrix
TCA
FA Oxidation
AA Oxidation
Electron Transport Chain (ETC)
moves electrons to O₂ and creates a H⁺ gradient
Succinate DH is __
complex 2
Electron Carriers include
NADH, FADH₂/FMDH₂, QH₂, Fe⁺², 2Cu⁺¹
NADH as Electron Carrier
carries 2 electrons
soluble in water
NADH → 2e⁻ + NAD⁺ + H⁺
FADH₂/FMDH₂ as an Electron Carrier
carries 2 electrons
always in a protein
FADH₂ → FADH• + 1e⁻ + 1H⁺ → FAD + 2e⁻ + 2H⁺
QH₂ as an Electron Carrier
carries 2 electrons
soluble in the membrane
QH₂ → QH• + 1e⁻ + 1H⁺ → Q + 2e⁻ + 2H⁺
Fe⁺² as an Electron Carrier
carries 1 electron as part of a heme of a F-S complex in protein
Fe⁺² → Fe⁺³ + e⁻
Cu⁺¹ as an Electron Carrier
carries 1 electron
in protein
2Cu⁺¹ → 2Cu⁺¹.5 + e⁻
Electrons are always carried __ during ETC
one at a time to O₂
Coenzyme Q (ubiquinore)
Hydrophobic
Soluble in membrane
Carries 2 electrons
Loosely bound to proteins
__ is loosely bound to proteins
Q
__ is tightly bound to proteins
Iron
ETC Complexes include
Complex 1, Complex 2: Succinate DH, Complex 3, Complex 4
Complex 2 includes what prosthetic group
FAD
Carriers within complexes
FMN, FAD (2e⁻)
Fe-S, Hemes, Cu (1 e⁻)
Carriers between complexes
QH₂: between 1 and 3, or between 2 and 3 (carries 2e⁻)
Cyt c: between 3 and 4 (carries 1 electron)
Either start at complex __ or __
1 or 2
If you start at complex 1, for every 1 NADH, you get __ H⁺ pumped
10
If you started at complex 2, for every succinate (FADH₂), you get __ H⁺ pumped
6
Complex 1 oxidizes
1 NADH
Complex 1 reduces
1 Q
Complex 1 pumps
4 H⁺ into the P site
Complex 2: Succinate DH oxidizes
1 Succiante
Complex 2: Succinate DH reduces
1 Q
Complex 2: Succinate DH
does not pump H⁺
__ is the ultimate produce of Succinate DH
QH₂.
FADH₂ is just an electron carrier within the protein
__from __ also reduces Q to QH₂ with no H⁺ pumped
FADH₂ from β-oxidation
Complex 3 oxidizes
1 QH₂
Complex 3 reduces
2 Cyt c
Complex 3 pumps
4 H⁺ to the P side
Complex 4 oxidizes
2 Cyt c
Complex 4 reduces
½ O₂
Complex 4 pumps
2 H⁺
Complex 4 prevents
electron transfer
Prevent electron transfer in Fe⁺³ from with
HCN
Prevent electron transfer in Fe⁺² form with
CO
ETC can lead to
oxidative damage(damage to DNA/proteins)
Reduction Potential (∆E°’)
the affinity a compound has for electrons (stability with electrons)
+∆E°’ = ∆G°’ ==
exergonic = more stable with electrons
During ETC, the electrons always
move to the molecule that wants them more
All complexes are
exergonic
ATP Synthesis is catalyzed by
Complex V (ATP Synthesis)
ATP Synthesis is dependent on
Presence of an H⁺ gradient (made by ETC)
Inner membrane impermeability
H⁺ movement through ATP Synthase (to make ATP)
(Part of Chemiosmotic Theory)
Complex V is made up of
FO , F₁, 𝛾
FO
in membrane
pumps H⁺
F1
in matrix
synthesizes ATP
𝛾
links the functions of FO and F₁
Stator
helps to stabilize FO and F₁
FO pumps H⁺ from P to N side and turns
itself and 𝛾 counterclockwise
F1 Synthesis of ATP
ADP + Pi → ADP + H₂O
Three identical sections (α/β) that are in three different conformations and move in a set order
The three conformations of (α/β) sections
Open (binds nothing)
Loose (binds ADP, Pi)
Tight (binds ATP)
The open conformation binds
nothing
The loose conformation binds
ADP, Pi (substrates)
The tight conformation binds
ATP
𝛾 forces ___ conformation and turns ___
O, counterclockwise
L is ___ from O
clockwise
T is __ from O
counterclockwise
__ H⁺ is pumped for every conformation change
3
It takes energy to ___ ATP
release (not make)
Takes __ H⁺ pumped from P to N to make 1 ATP
4
To release ATP, it takes __ H⁺
3
To transport Pi into the matrix, it takes __ H⁺
1
P/O ratio is
the ATP yield from the reduction of ½ O₂
NADH to ½ O₂ gets __ H⁺
10
FADH₂ to ½ O₂ gets __ H⁺ pumped
6
NADH P/O Ratio
2.5 ATP
FADH₂ P/O Ratio
1.5 ATP
There is ___ regulation of Oxidative Phosphorylation
little
The overall rate of oxidative phosphorylation depends on
substrate availability and
cellular energy demand (no allosteric control)
Important Substrates for Oxidative Phosphorylation
NADH, Succinate (FADH₂) O₂, ADP and Pi
We need __ the substrates for oxidative phosphorylation to work
all
The ______ couples the electron transport chain to ATP Synthesis
proton gradient
____ move H⁺ across the inner membrane
Uncouplers
Uncouplers uncouple oxidative phosphorylation by
destroying the proton gradient
In the presence of uncouplers, the body is
breaking down compounds for energy, but little ATP is made (lots of heat instead)
Uncouplers move H⁺ across the membrane using
Proton Transporter (neutral)
Membrane soluble compound
CO inhibits
Complex IV of ETC
The rate-limiting step is
the release of ATP into the matrix (tight to open)
Water Molecules Released During ATP synthase
(# of total molecules of NADH and FADH₂) + (# of NADH x 2.5)+ (# of FADH₂ x 1.5)
NADH electrons Transport To Ox Phos
First, they are transferred to OAA to make Malate (via cytosolic malate DH).
Then, malate passes through the mitochondrial membrane to the matrix
Once in the matrix, the electrons then pass from Malate to OAA to NAD⁺ to make a new NADH (uses mitochondrial malate DH).
Then they can react with complex 1 in the ETC
Pathway for Electrons from NADH through electron transport
go into complex 1 as Q to make QH₂ (4 protons are pumped to the P side)
QH₂ goes into complex 3, where the two electrons are transferred to make two reduced Cyt c (4 protons are pumped)
The two Cyt c go into Complex 4, and the two electrons are then transferred to ½ O₂ to make one H₂O (2 protons are pumped)
A total of 10 protons are pumped.