Chemistry Review
1/16
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
How do you calculate the formal charge of an atom?
Formal charge = (Valence electrons) - (Nonbonding electrons) - 1/2(Bonding electrons)
Which ions have the same electron configuration as Ne?
Na+, Mg2+, and O2- all have the same electron configuration as Ne: 1s² 2s² 2p⁶
What is the shape of a p orbital?
Dumbbell-shaped with a nodal plane at the nucleus
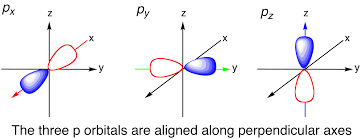
What hybridization leads to linear geometry?
sp
What orbitals overlap to form a sigma bond in ethane?
sp3-sp³
Is the C=O bond polar?
Yes, oxygen is more electronegative, so it bears a partial negative charge

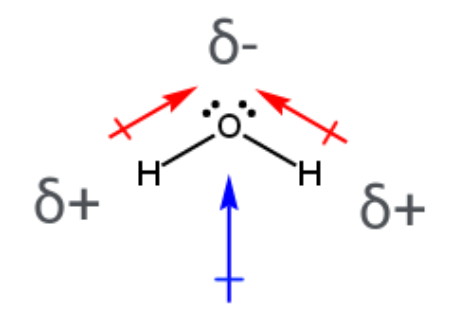
What determines if a molecule has a dipole moment?
Asymmetrical geometry and polar bonds lead to a dipole moment
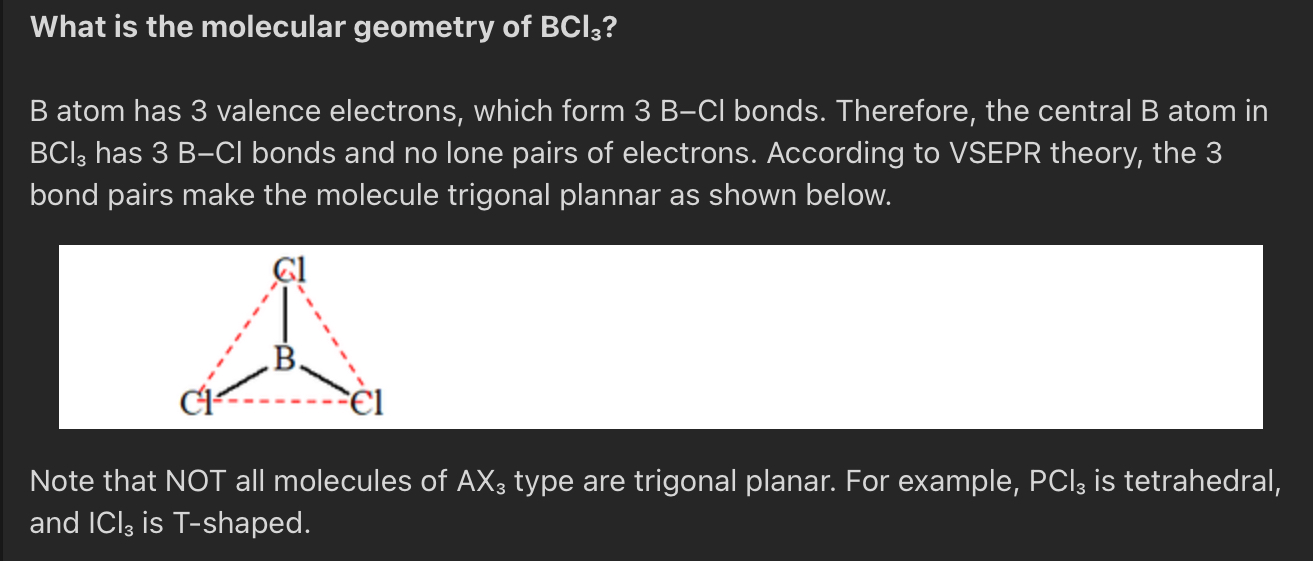
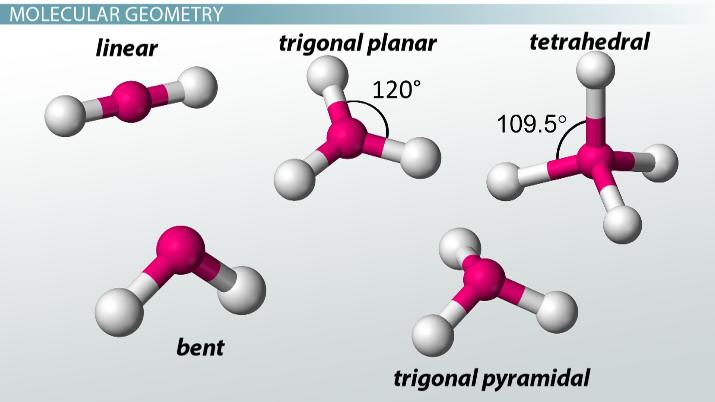
What geometry does BCl₃ have?
Trigonal planar
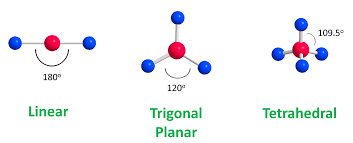
What geometry does NH₃ have?
Trigonal pyramidal
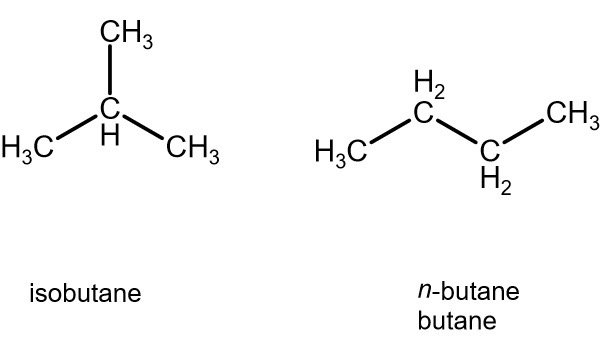
Why does isobutane have a lower boiling point than n-butane?
Isobutane has weaker van der Waals dispersion forces due to less surface area
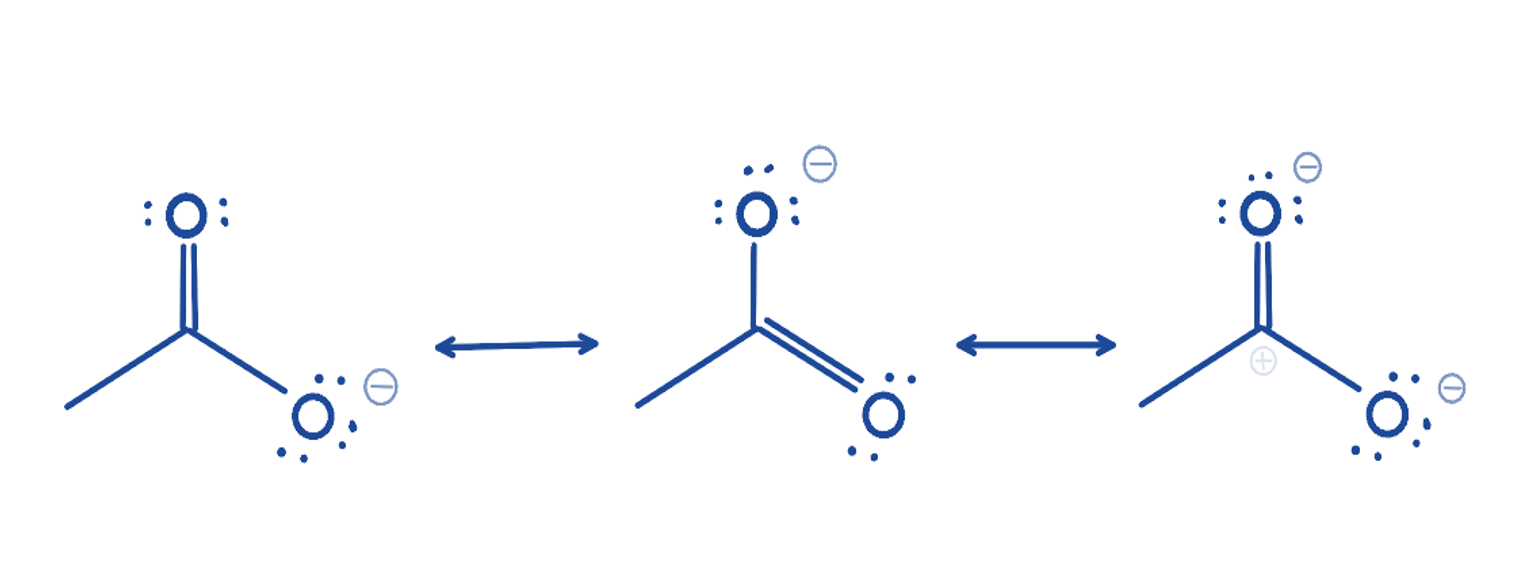
What is a resonance structure?
A different valid Lewis structure for the same molecule showing delocalization of electrons
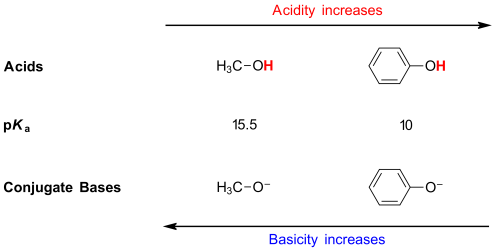
What factors affect acidity?
Resonance stabilization, electronegativity, atom size, and inductive effects
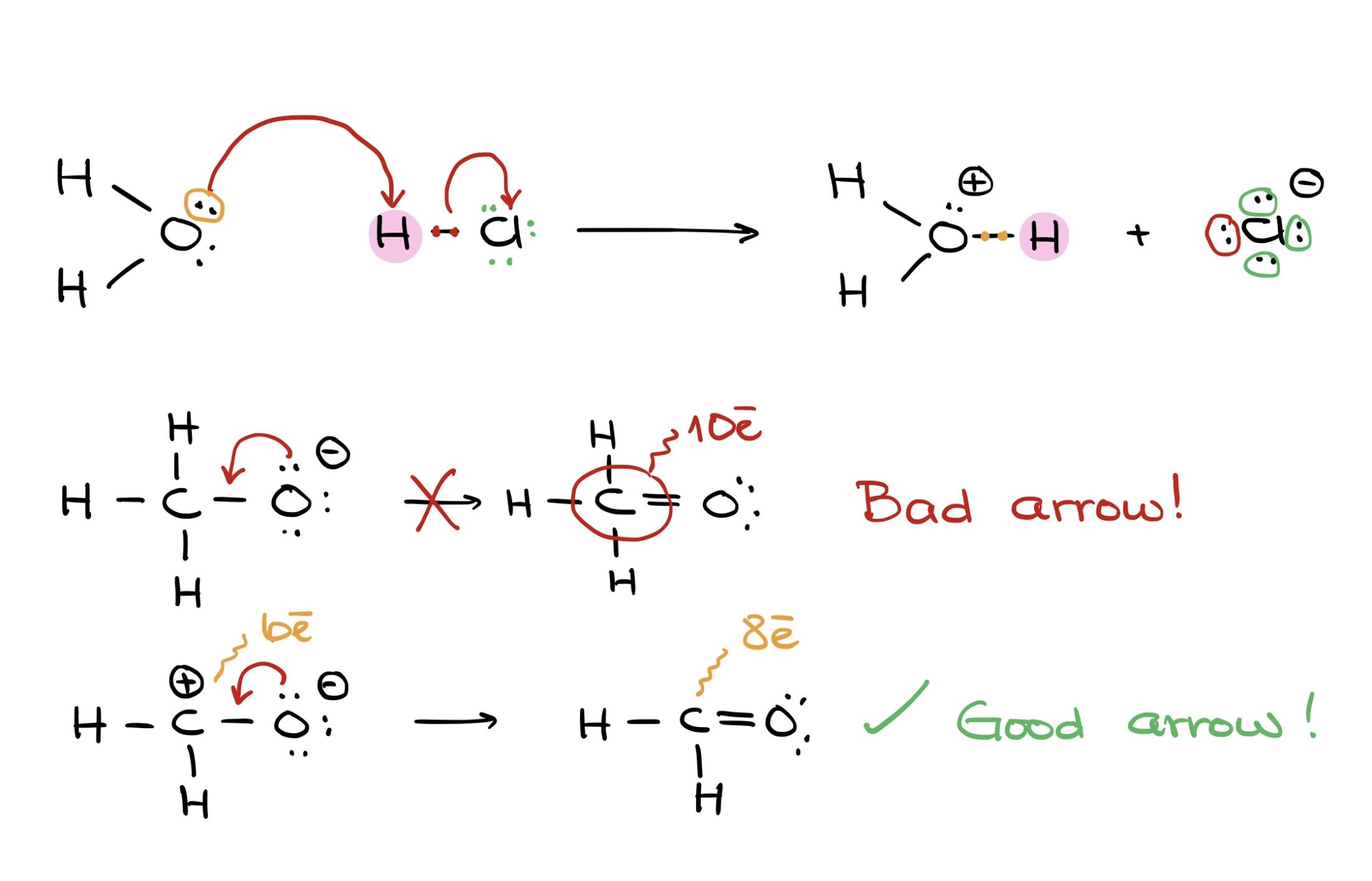
What do curved arrows represent in reaction mechanisms?
Movement of electron pairs from a nucleophile to an electrophile or bond
What is the bond angle in CH₄?
109.5° (tetrahedral)
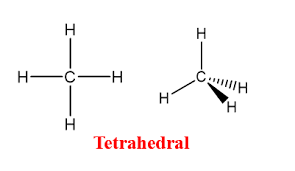
What is the bond angle in CH₂=CH₂?
120° (trigonal planar)
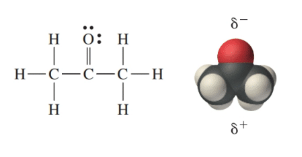
What is the Lewis structure of acetone (CH₃COCH₃)?
CH₃-C(=O)-CH₃, with the carbonyl carbon double-bonded to oxygen
What are constitutional isomers?
Compounds with the same molecular formula but different connectivity of atoms
