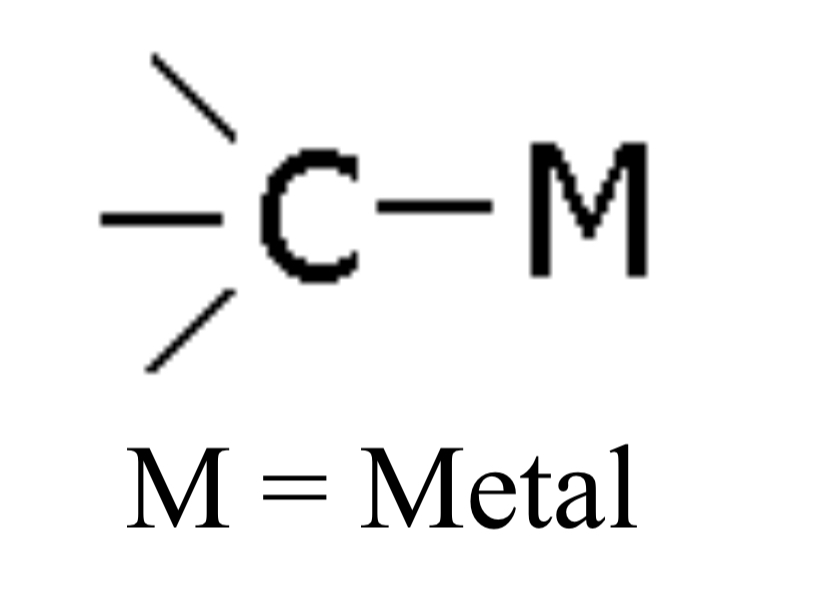Functional Groups Flashcards
1/15
Earn XP
Description and Tags
Flashcards for reviewing functional groups and their naming conventions in organic chemistry.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Alkene
Compound with a double bond, ending in -ene (e.g., Propene)
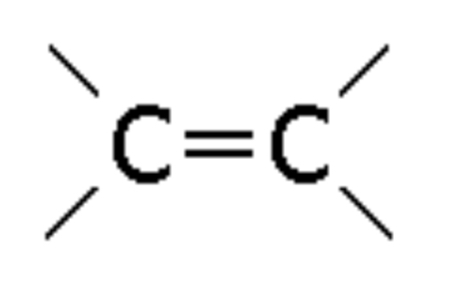
Alkyne
Compound with a triple bond, ending in -yne (e.g., Propyne)
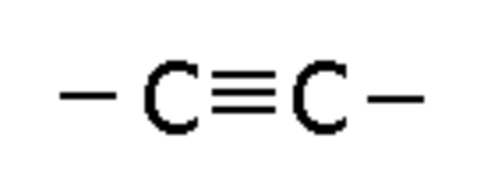
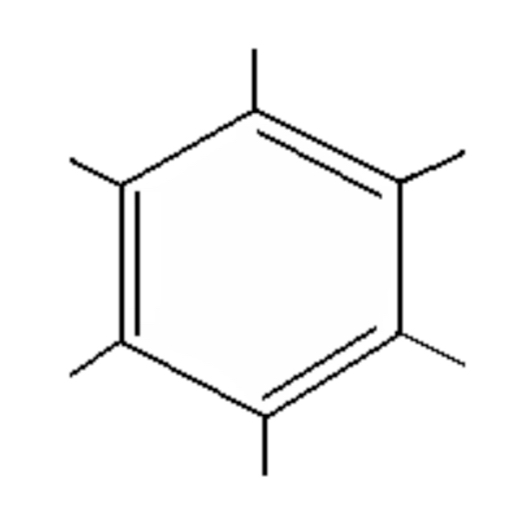
Arene
Aromatic compound (e.g., Benzene), no specific ending
Halide
Compound with a halogen (F, Cl, Br, I, e.g., Iodoethane), no specific ending
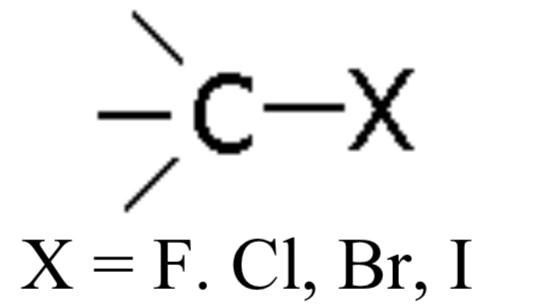
Alcohol
Compound with -OH group, ending in -ol (e.g., Ethanol)
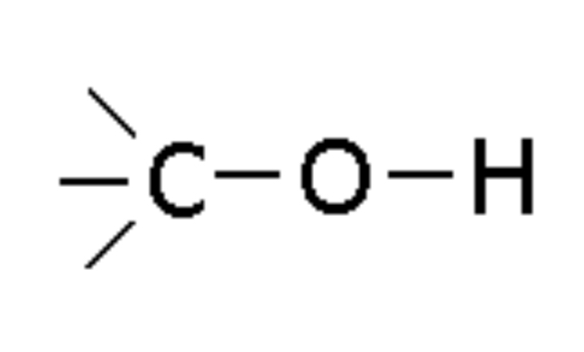
Ether
Compound with -O- linkage (e.g., Ethoxyethane or Diethyl ether), named as ether
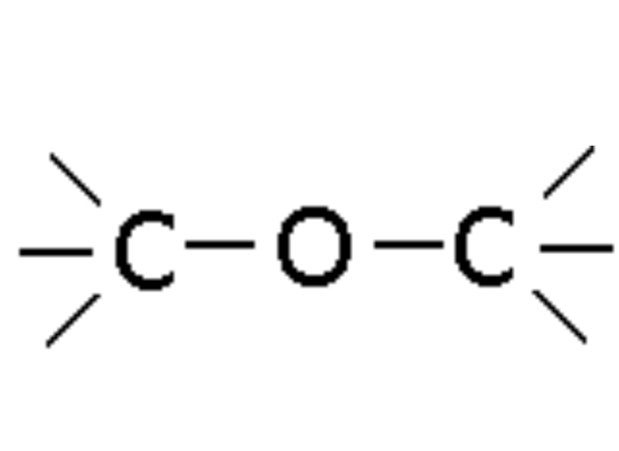
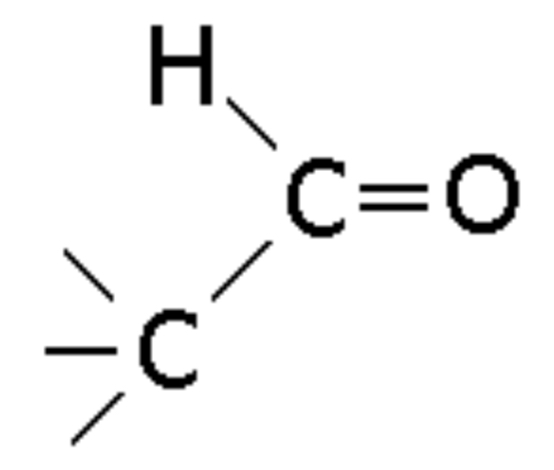
Aldehyde
Compound with a carbonyl group at the end of the chain, ending in -al (e.g., Ethanal)
Ketone
Compound with a carbonyl group in the middle of the chain, ending in -one (e.g., 2-Propanone)
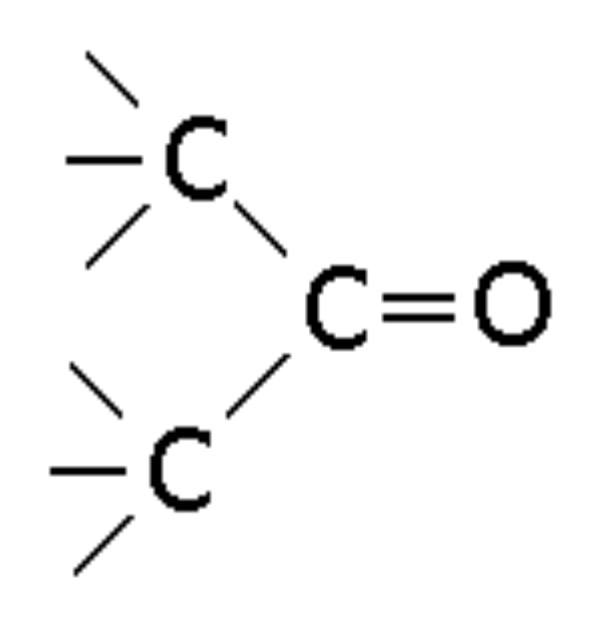
Ester
Compound with -COOC- linkage, ending in -oate (e.g., Ethyl Ethanoate)
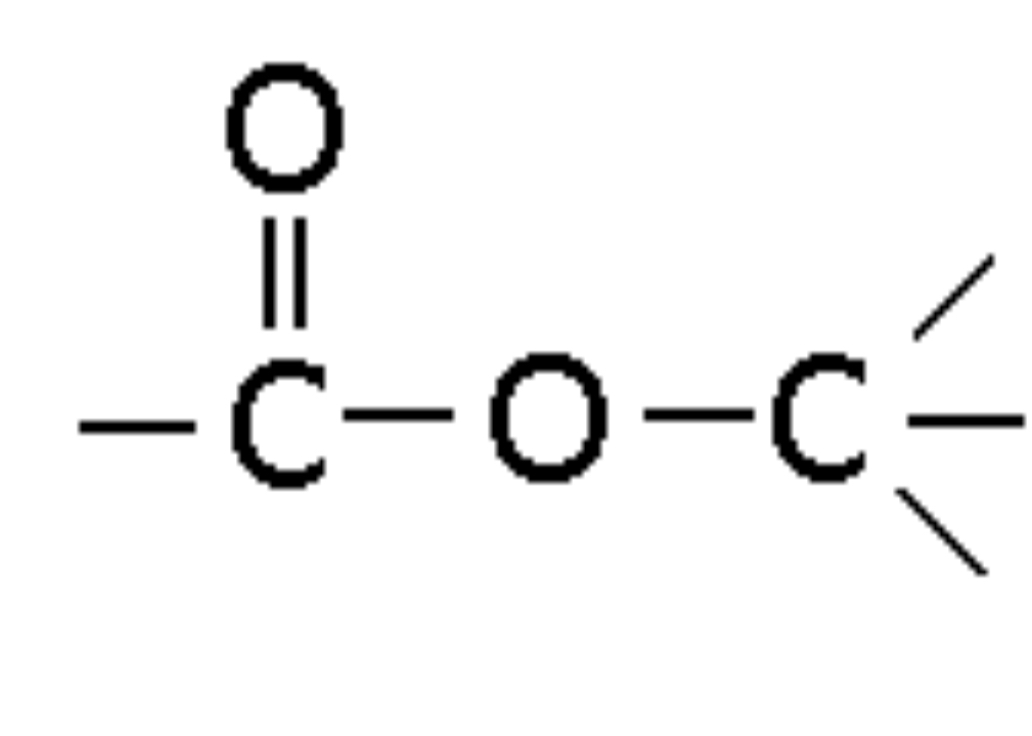
Carboxylic Acid
Compound with -COOH group, ending in -oic acid (e.g., Butanoic acid)
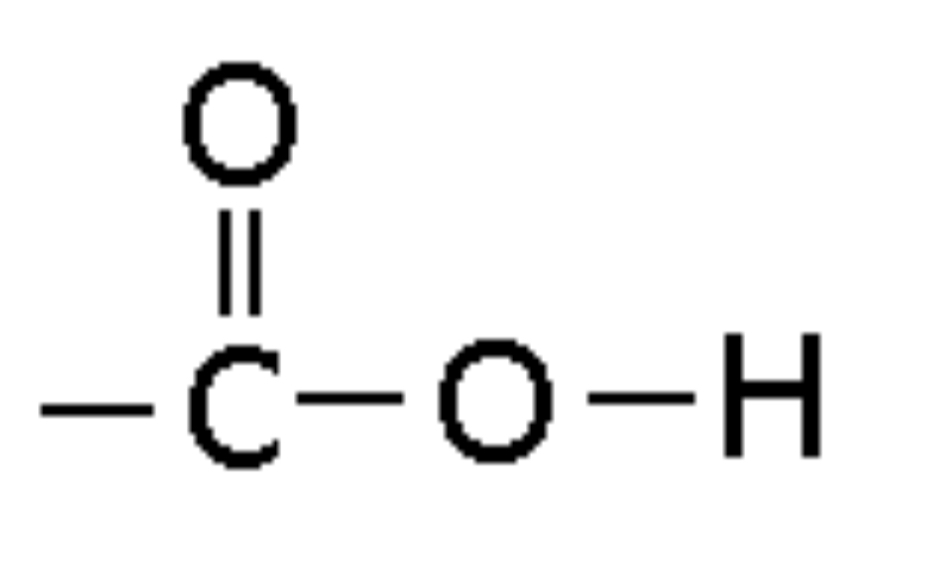
Amide
Compound with -CON- linkage, ending in -amide (e.g., Ethanamide)
Amine
Compound with -NH2 group, ending in -amine (e.g., Ethylamine)
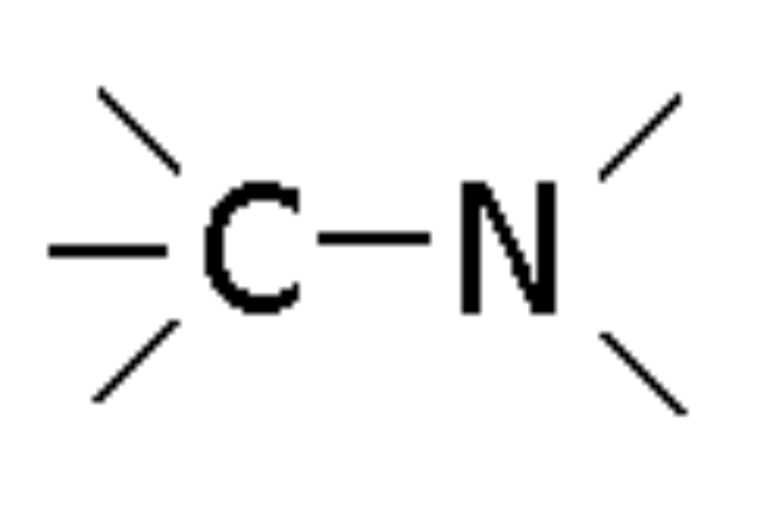
Nitrile
Compound with -CN group, ending in -nitrile (e.g., Ethanenitrile)
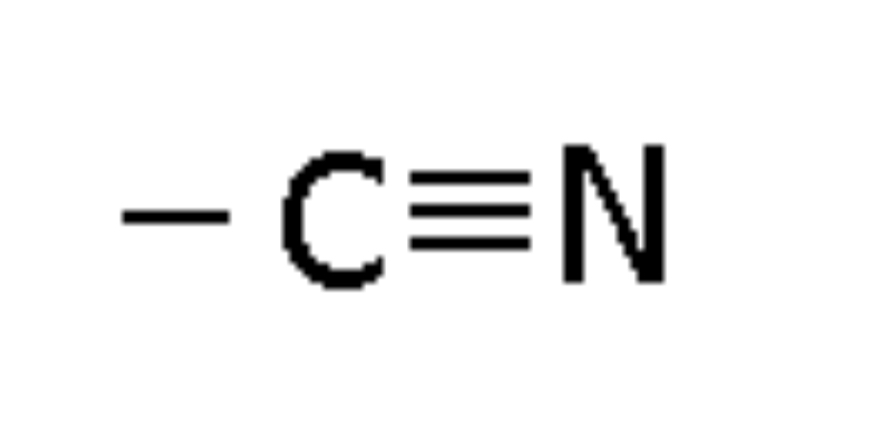
Nitro
Compound with -NO2 group, using nitro as a prefix (e.g., Nitroethane)

Thiol
Compound with -SH group, named thiol (e.g., Methyl thiol)
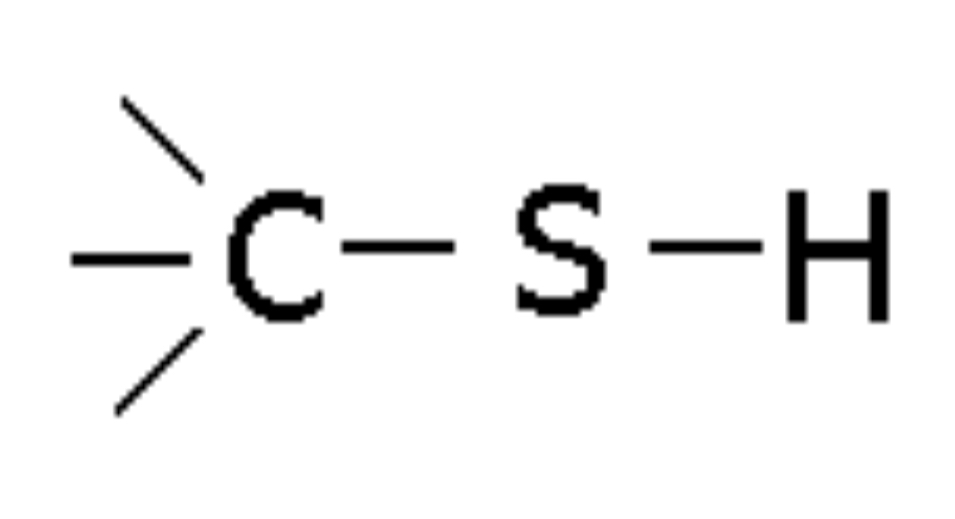
Organometallic
Compound with a metal-carbon bond (e.g., Methyl lithium), no specific ending