CHM 205 Lab Final Studying
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
Melting point determination
An intensive property, for a pure compound, melting point can be used to identify the compound
Fast melting point determination
Heating rate of 10°C per minute.
Slow melting point determination
Heating rate of 2°C per minute.
From the slow melting point determination, looking at where the compound is closest to that range, and test under it.
Mixed melting point determination
Put the same small amount of unknown substances and one of your two possible substances on the same watch glasses.
Mix them together.
Take the melting point, if the melting point match from prior, you can identify the substance.
Gravity filtration
Best for granular substances
Example: Removing a drying agent from a 50 mL of a solution
Overall rather slow

Why is fluted filter paper used in gravity filtration?
Results in a larger surface area (higher yield) and faster filtration
Recrystallization
Used to purify solid compounds. Involves the dissolving of the impure compound in a hot solvent and then slowly cooling the solution
If a concentrated hot solution is allowed to cool, crystal start to form. If crystal formation is slow and well-controlled, the crystal will only allow the same molecules to be incorporated in the crystal structure, excluding the molecules that do not fit in.
Comes down to "segregation" on a molecular level
Cooling the solution in an ice bath or leaving it in the refrigerator or freezer overnight can lead to good yields of recovery.
Recrystallization laboratory technique
Dissolve the impure solid in a minimum amount of solvent at a high temperature.
Filter the solution while hot if there is an insoluble impurity.
Cool the solution slowly without disturbing the crystallization process.
Dry the crystals.
Different recrystallization scenarios
Purification by recrystallization can be successful when there is a large difference in solubility between the compound of interest and the impurity.
What was the purpose of using the charcoal pellets in the recrystallization experiment?
Purpose is to remove the colored impurities within the sample by adsorbing them. Removed through filtration process.
The solubility of an unknown compound X in ethanol is found to be 0.21 grams per 10 mL at 0°C and 1.4 grams per 10 mL at 78°C. What is the minimum amount of ethanol needed to dissolve a 2.0 gram sample of compound X at 78°C?
Known: Solubility of compound X in ethanol is 1.4 grams per 10 mL at 78°C.
Ratio of solubility to volume: 1.4 grams / 10 mL = 0.14 g/mL
Volume of ethanol needed = Mass / solubility = 2.0 grams / 0.14 g/mL = 14.29 mL
After recrystallization of the 2.0 gram sample in an ice bath, how much of compound X will remain dissolved in the cooled solvent? (not recovered)
Ratio of solubility to volume: 0.21 grams / 10 mL = 0.021 g/mL
Amount of compound X remaining = 0.021 g/mL * 14.29 mL = 0.3 g
Rank the solvents from most to least polar: acetone, hexane, water.
Water (most)
Extremely polar because of its strong hydrogen bonding.
Acetone
Polar due to carbonyl group (carbon double bonded to oxygen)
Hexane (least polar)
Nonpolar structure is made up entirely of carbon and hydrogen in a straight chain.
Is acetone miscible or immiscible?
Acetone is miscible with water and many other organic solvents (because it is polar enough to mix with water, but also has non polar parts).
Is hexane miscible or immiscible?
Immiscible with water (nonpolar, does not mix with polar solvents like water)
Is water miscible or immiscible?
Miscible with other polar solvents (like alcohols, acetone, etc.) but immiscible with nonpolar ones (like hexane)
Like dissolves like, polar mixes with polar, nonpolar with nonpolar!
How do you take a proper melting point?
Finely crush the sample (powder it), pack a tiny amount (1-2 mm high) into a capillary tube, insert the tube into the melting point apparatus, start heating slowly (especially as you approach the expected melting point), watch carefully for when the first drop of liquid appears (start) and when the entire sample becomes liquid (end).
What is the proper heating rate?
About 1-2°C per minute near the expected melting point.
Can you re-use melting point samples?
No you should NOT reuse them. Once a sample melts, it might decompose, absorb water, or change structure when it cools - so it won't give an accurate melting point again.
How are colored impurities removed during recrystallization?
You add a small amount of activated charcoal (aka decolorizing carbon) to the hot solution. The charcoal adsorbs the colored impurities. Then you quickly filter the hot solution to remove the charcoal (and the attached impurities) before recrystallization.
Suppose you have 500 mg of an organic compound fully dissolved in 2 mL of hot water. If you cool to 25°C, and the solubility of the compound at 25°C is 50 mg/ml, how much of the compound should you recover?
Recovered = original amount - amount remaining dissolved
At 25°C, 2 mL * 50 mg/mL = 100 mg stays in the dissolved water.
Recovered = 500 mg - 100 mg = 400 mg, recover 400 mg after recrystallization
Partition coefficient calculation
C2/C1
C2 = The concentration of the solute in solvent 2
C1 = the concentration of the same solute in solvent 1 in g/L
Liquid liquid extraction
Requires two immiscible liquids (aqueous & organic phase) use separatory funnel; lower density on the bottom; like dissolves like; organic non-polar compounds will dissolve in organic phase
Basic principles of liquid-liquid extraction
A mixture of two compounds, one water-soluble and one water-insoluble, can be separated by adding both water and an organic solvent.
The water-soluble component is soluble in the water phase, and the other component is soluble in the organic solvent.
If the organic solvent and the water are immiscible, the two layers can be separated, thereby accomplishing the separation of the two compounds.
Drying agent rules
1. Only dry the organic layer, NOT the aqueous layer
2. Choose an appropriate drying agent (anhydrous sodium sulfate vs calcium chloride)
3. Ensure the organic layer is mostly free of visible water droplets first
4. Add the drying agent slowly and in small amounts
5. Filter the solution after drying
Suppose that just before adding a drying agent to an organic solvent that was used to extract an aqueous solution, you notice that there are still tiny water droplets in the organic layer. What should you do?
a. Proceed with adding the drying agent — it will remove the water droplets.
b. Shake the flask harder to mix the layers together
c. Do not add the drying agent; instead, evaporate some water in the fume hood using air plugs and ensure the layers are properly separated.
d. Add more organic solvent to dilute the water droplets.
c. Do not add the drying agent; instead, evaporate some water in the fume hood using air plugs and ensure the layers are properly separated.
What is the correct general extraction scheme for separating an organic base (B) mixed with a neutral organic compound (N)?
a. Add sodium hydroxide to form a water-soluble base and leave the neutral compound in the organic phase.
b. Add dilute hydrochloric acid to protonate the base into a water-soluble form, leaving the neutral compound in the organic phase.
c. Add water and shake to dissolve both into the aqueous phase.
d. Add more organic solvent to force separation by solubility differences.
b. Add dilute hydrochloric acid to protonate the base into a water-soluble form, leaving the neutral compound in the organic phase.
What is the correct general extraction scheme for separating an organic acid (HA) from a phenol (ArOH)?
a. Add sodium hydroxide to deprotonate the phenol into a water-soluble form, leaving the organic acid behind.
b. Add dilute hydrochloric acid to neutralize both compounds into the organic phase.
c. Add sodium hydroxide to deprotonate the organic acid into a water-soluble form, leaving the phenol in the organic phase.
d. Add water and shake vigorously to separate them based on density.
c. Add sodium hydroxide to deprotonate the organic acid into a water-soluble form, leaving the phenol in the organic phase.
If you are unsure which layer is the aqueous layer during a liquid-liquid extraction, what simple method could you use to figure it out?
a. Smell each layer to see which one smells like water.
b. Add a small amount of water and observe which layer the water mixes with and causes an increase in volume.
c. Shake the container vigorously and see which layer disappears.
d. Evaporate both layers and see which one leaves no residue.
b. Add a small amount of water and observe which layer the water mixes with and causes an increase in volume.
Which gives the most efficient extraction scheme for step 1?
a. Extract one time with 4.5 mL NaHCO3
b. Extract twice with 2.25 mL each
c. Extract three times with 1.5 mL each time
c. Extract three times with 1.5 mL each time
Suppose you have mixture of acid/phenol/amine in MtBE. What reagent would you use to extract only the amine?
a. Na2SO4
b. NaHCO3
c. HCl
d. NaOH
e. MtBE
c. HCl
This is about what will protonate/deprotonate ONLY the functional group you want. I.e. need an acid to extract a base, and a base to extract an acid
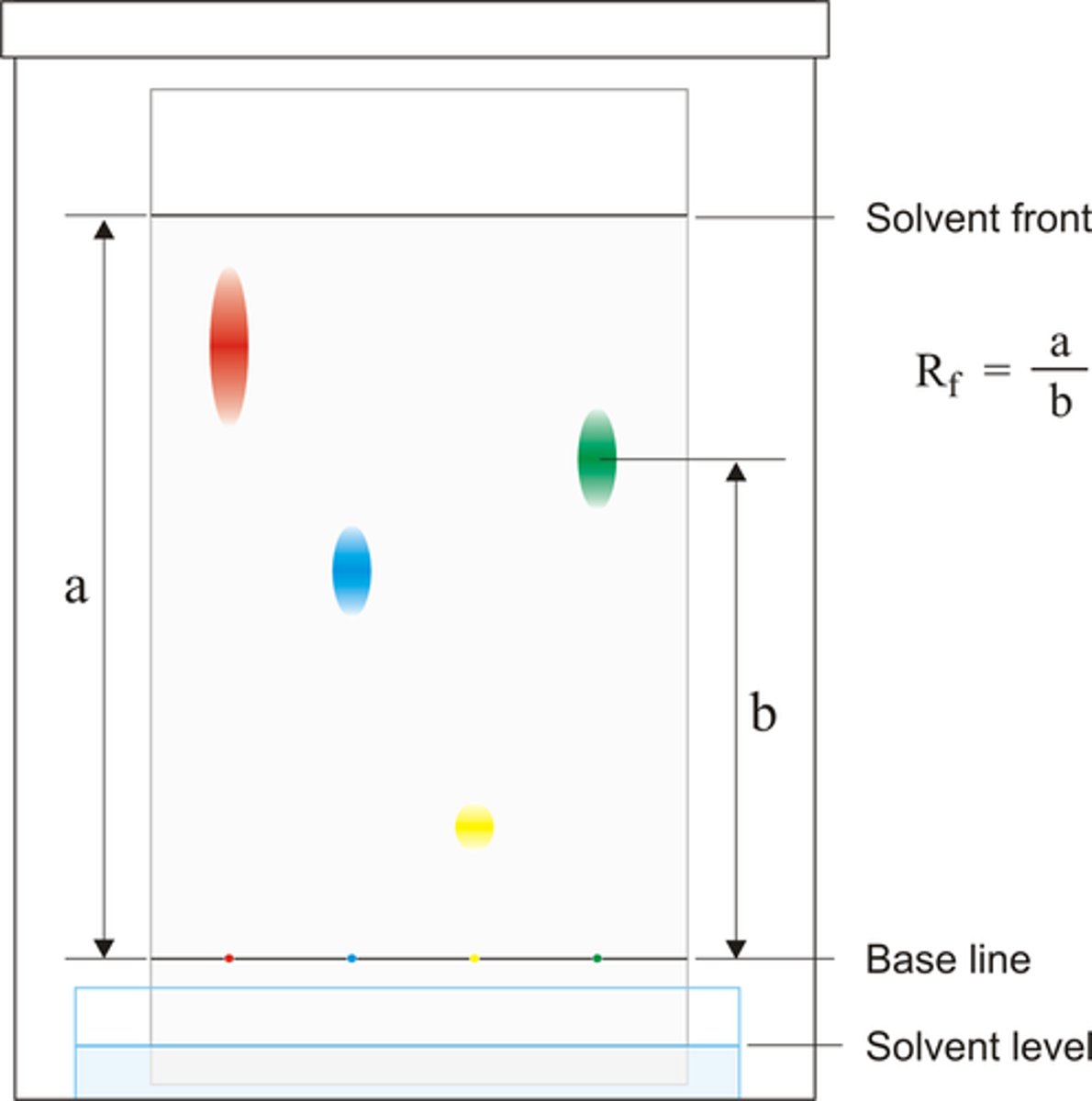
Rf values
Distance travelled by substance / distance travelled by solvent
Always between 0-1
Higher Rf value
Less polar
Lower Rf value
More polar
Which statement best explains why p-hydroxyacetophenone is more polar than o-hydroxyacetophenone?
A) The carbonyl group is closer to the OH group in p-hydroxyacetophenone, increasing polarity.
B) The OH group in p-hydroxyacetophenone is positioned further from the carbonyl group, allowing for more hydrogen bonding with silica.
C) o-Hydroxyacetophenone forms stronger hydrogen bonds with silica due to the close proximity of its functional groups.
D) The difference in polarity is due to the presence of an extra OH group in p-hydroxyacetophenone.
B) The OH group in p-hydroxyacetophenone is positioned further from the carbonyl group, allowing for more hydrogen bonding with silica.
Which of the following correctly ranks ferrocene, acetylferrocene, and diacetylferrocene in order of increasing polarity?
A) Acetylferrocene < Ferrocene < Diacetylferrocene
B) Ferrocene < Acetylferrocene < Diacetylferrocene
C) Diacetylferrocene < Acetylferrocene < Ferrocene
D) Ferrocene < Diacetylferrocene < Acetylferrocene
B) Ferrocene < Acetylferrocene < Diacetylferrocene
Why does o-hydroxyacetophenone have a much lower melting point than p-hydroxyacetophenone?
a) It forms stronger intermolecular hydrogen bonds.
b) It forms intramolecular hydrogen bonds, weakening crystal packing
c) It has a larger molecular weight
d) It has a more symmetric structure
b) It forms intramolecular hydrogen bonds, weakening crystal packing
You run a TLC using ethyl acetate and observe only one spot with a Rf of .91 for a mixture of two compounds. What change should you make?
a) Use a more polar solvent.
b) Use a less polar solvent
c) Use a larger TLC plate
d) Increase the spotting concentration
b) Use a less polar solvent
What happens if the solvent level in the TLC chamber is above the spots?
a) The solvent evaporates too quickly
b) The Rf value becomes larger
c) The spots dissolve into the solvent
d) The TLC plate will crack
c) The spots dissolve into the solvent
What compound would you expect to have the highest Rf value?
a) O-toluic acid
b) Fluorenol
c) Naphthalene
c) Naphthalene
Which compound would have the larger Rf value on a SiO2 TLC plate in 10% acetone/hexane?
a) 4-decanone
b) 4-decanol
a) 4-decanone
Arrange the following solvents in order of increasing polarity (least to most):
a) Heptane, toluene, dichloromethane, ethyl acetate, isopropanol, ethanol
b) Ethanol, isopropanol, ethyl acetate, dichloromethane, toluene, heptane
c) Heptane, ethyl acetate, dichloromethane, toluene, isopropanol, ethanol
d) Toluene, heptane, dichloromethane, ethyl acetate, isopropanol, ethanol
a) Heptane, toluene, dichloromethane, ethyl acetate, isopropanol, ethanol
What is not important when spotting a TLC plate?
a) Label each spot
b) Spot heavily to ensure the compound shows up
c) Spot at least 1 cm above the bottom of the plate
d) Use small, concentrated spots
b) Spot heavily to ensure the compound shows up
How does TLC show that column chromatography successfully purified a crude sample?
a) Crude sample TLC has many spots, purified sample has one
b) Crude sample TLC is blank, purified has many spots
c) Crude sample runs higher than purified sample
d) Purified sample shows a larger Rf than the crude
a) Crude sample TLC has many spots, purified sample has one
If a more polar solvent is used on a TLC plate, how would the Rf value change?
a) They would decrease
b) They would increase
c) They would stay the same
d) They would become negative
b) They would increase
Why should the sample not be diluted before loading onto a chromatography column?
a) To make the column taller
b) To avoid cracking the silica
c) To save solvent
d) To prevent smearing and ensure sharp separation
d) To prevent smearing and ensure sharp separation
Which compound would elute first from the chromatograph column?
a) Diacetylferrocene
b) Acetylferrocene
c) Ferrocene
c) ferrocene
You run a column on a thiol and an amine using petroleum ether and dichloromethane and nothing elutes. What is the best correction?
a) Decrease solvent polarity
b) Increase solvent polarity
c) Switch to hexane
d) Dry the column before running again.
b) Increase solvent polarity
When separating naphthalene, o-toluic acid, and fluorenol by column chromatography, what is the elution order?
a) Fluorenol → Naphthalene → o-Toluic acid
b) o-Toluic acid → Fluorenol → Naphthalene
c) Naphthalene → Fluorenol → o-Toluic acid
d) Naphthalene → o-Toluic acid → Fluorenol
c) Naphthalene → Fluorenol → o-Toluic acid
Thin layer chromatography can be used in many ways, but which of the following is NOT an application of thin layer chromatography?
a) Select a solvent system for column chromatography
b) Monitor the progress of a reaction
c) Separation and identification of volatile compounds
d) ID an unknown compound by comparison to standards
c) Separation and identification of volatile compounds
What is a likely consequence of overloading a TLC (Thin Layer Chromatography) spot?
A) Faster elution and sharper spots
B) No movement of the sample up the plate
C) Dragging or smearing up the plate, leading to poor separation
D) Complete evaporation of the sample before development
C) Dragging or smearing up the plate, leading to poor separation
What is a likely consequence if a chromatography column runs dry during the separation?
A) Faster elution and better separation
B) Cracks form in the stationary phase, leading to poor separation
C) Increased polarity of the stationary phase
D) Improved resolution between compounds
B) Cracks form in the stationary phase, leading to poor separation
Which of the following could cause two compounds to not separate properly during chromatography?
A) Incorrect solvent choice
B) Very similar polarities between the compounds
C) Poor column packing
D) All of the above
D) All of the above
Thin layer chromatography
a separation technique that involves the separation of small molecules as they move through a silica gel (polar)
Very rapid separation of small amounts of material, thin coating of silicia gel on plate of glass is used as the stationary phase (polar) allowing for the moving liquid to climb up the plate
TLC procedure major steps
1. Injection: For the TLC the term "spotting" is used; the sample is applied to the plate before any solvent is allowed to ascend the adsorbent layer.
2. Separation: Developing or "running" the plate; as the solvent ascends the plate, the plate sample is partitioned between the moving liquid phase and the stationary solid phase.
3. Detection: Different methods may be used to visualize the separated spots.
Analysis of TLC
Rf values:
- If two compounds have the same Rf value running side by side on a plate, there is a good chance that these are the same compounds.
- If two compounds have different Rf values running side by side on the same plate, it is almost certain that these are different compounds.
Which compound travels up a silicia gel plate faster?
More polar compounds have a greater affinity for the polar stationary phase, resulting slower movement up the plate.
Therefore, less polar compounds travel up the plate faster (higher Rf)
Which was more polar, the ortho or para isomer of hydroxyacetophenone?
The para isomer was more polar, therefore it should have traveled a shorter distance up the plate as compared to the ortho isomer.
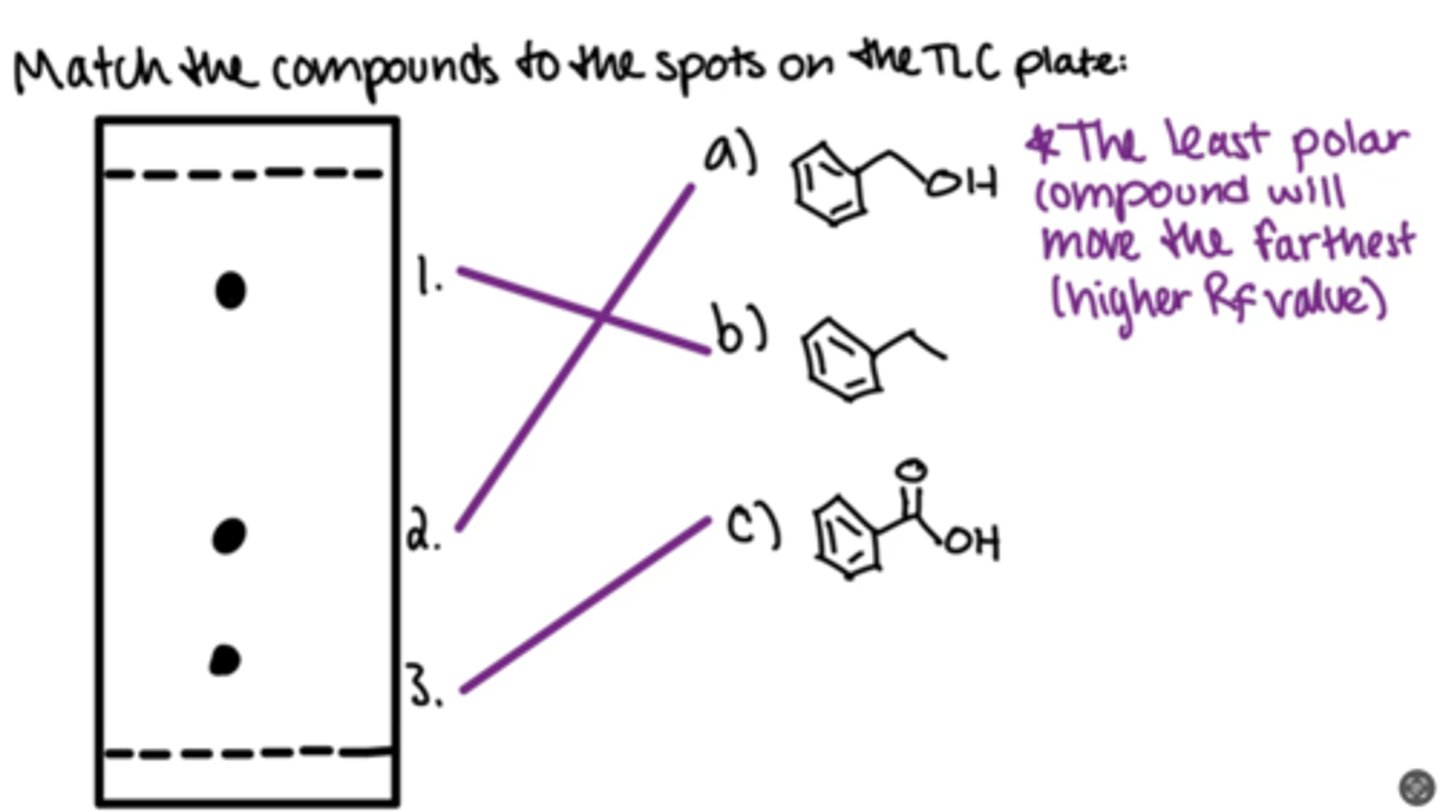
Match the compounds to the spot on the TLC silica gel plate
The least polar compound will move the farthest (higher Rf value)
Carboxylic acid is more polar than an alcohol, which is more polar than an alkane
The stationary phase in your column is:
a) Polar
b) Neutral
c) Non-polar
a) Polar
What is the stationary phase made of?
a) Sodium chloride
b) Silica gel
c) Acetone
d) Benzene
b) Silica gel
The mobile phase in your column is:
a) Polar
b) Neutral
c) Non-polar
c) Non-polar
How do you determine which compound would elute first in column chromatography based on polarity?
a) The compound with the highest polarity elutes first
b) The compound with the lowest polarity elutes first.
c) The compound with the highest molecular weight elutes first
d) The compound with the most functional groups elutes first
b) The compound with the lowest polarity elutes first.
Which would elute from your column last?
a) Carboxylic acid
b) Halocarbon
c) Alcohol
d) Ketone
a) Carboxylic acid
Which would elute from your column first?
a) Carboxylic acid
b) Halocarbon
c) Alcohol
d) Ketone
b) Halocarbon
Which distillation method requires a larger difference in boiling points between components, typically greater than 25°C?
A) Simple Distillation
B) Fractional Distillation
C) Steam Distillation
D) None of the above
A) Simple Distillation
Which distillation technique is most effective for separating mixtures with close boiling points (less than 25°C difference)?
A) Simple Distillation
B) Fractional Distillation
C) Steam Distillation
D) All of the above
B) Fractional Distillation
Which distillation method is commonly used for mixtures of volatile liquids and water?
A) Simple Distillation
B) Fractional Distillation
C) Steam Distillation
D) Both A and C
C) Steam Distillation
Which distillation method is typically used for temperature-sensitive substances due to its ability to lower the boiling point using steam?
A) Simple Distillation
B) Fractional Distillation
C) Steam Distillation
D) All of the above
C) Steam Distillation
Under what conditions can a good separation be achieved by simple distillation?
A) The two components must have a boiling point difference of at least 5°C, and both components must decompose at their boiling points.
B) A high difference in boiling points (at least 25-30°C) between the components, and both components must not undergo thermal decomposition at their boiling points.
C) The components must have identical boiling points and must not decompose at their boiling points.
D) The mixture must contain more than two components for simple distillation to be effective.
B) A high difference in boiling points (at least 25-30°C) between the components, and both components must not undergo thermal decomposition at their boiling points.
Why does the boiling point of a cyclohexane: toluene mixture rise slowly throughout a simple distillation procedure?
A) The boiling point rises quickly due to the vapor pressure of cyclohexane decreasing.
B) As distillation progresses, the mole fraction of cyclohexane increases, leading to a steady decrease in the boiling point.
C) As the distillation progresses, the mole fraction of toluene in the remaining liquid increases, leading to a steady increase in the boiling point as the mixture becomes richer in the higher-boiling component (toluene).
D) The boiling point rises slowly because both cyclohexane and toluene have similar boiling points and distill at the same rate.
C) As the distillation progresses, the mole fraction of toluene in the remaining liquid increases, leading to a steady increase in the boiling point as the mixture becomes richer in the higher-boiling component (toluene).
Why do we use steam distillation to isolate eugenol rather than purify it by simple distillation?
A) Steam distillation is used because eugenol has a low boiling point and can easily be distilled at temperatures below 100°C.
B) Steam distillation lowers the boiling point of eugenol, allowing isolation at a safer temperature (~100°C), preventing decomposition.
C) Steam distillation is preferred because eugenol decomposes at temperatures below 100°C, making simple distillation unsafe.
D) Simple distillation is always preferred for isolating eugenol because it allows for better purity.
B) Steam distillation lowers the boiling point of eugenol, allowing isolation at a safer temperature (~100°C), preventing decomposition.
Does Zaitsev's rule come into play during the E1 reaction carried out in this experiment 5? Why or why not?
a. Yes, Zaitsev's rule is applicable because the reaction involves the dehydration of cyclohexanol, which produces a more substituted and stable alkene. However, the rule doesn't significantly affect the outcome because only one possible alkene (cyclohexene) is formed.
b. No, Zaitsev's rule doesn't apply because cyclohexanol doesn't undergo dehydration to form an alkene.
c. Yes, Zaitsev's rule applies and the reaction favors the formation of the least substituted alkene.
d. No, Zaitsev's rule doesn't apply because the reaction produces a single alkene regardless of substitution.
a. Yes, Zaitsev's rule is applicable because the reaction involves the dehydration of cyclohexanol, which produces a more substituted and stable alkene. However, the rule doesn't significantly affect the outcome because only one possible alkene (cyclohexene) is formed.
The E1 mechanism is a one-step mechanism. True or false?
a. True, the E1 mechanism is a single-step elimination reaction.
b. False, the E1 mechanism is a stepwise elimination reaction.
c. True, the E1 mechanism involves simultaneous bond-breaking and bond-forming.
d. False, the E1 mechanism is a concerted mechanism where all steps occur simultaneously.
b. False, the E1 mechanism is a stepwise elimination reaction.
What is the mechanism for the acid-catalyzed E1 reaction of 2-butanol?
a. The E1 mechanism starts with the protonation of the hydroxyl group by acid, converting it into a better leaving group (water). Water departs, forming a carbocation, followed by the removal of a proton from a beta-carbon by a base, leading to the formation of 2-butene.
b. The E1 mechanism begins with the elimination of water without any protonation, leading to the formation of a primary carbocation, which is unstable and undergoes rearrangement.
c. The reaction proceeds via a one-step concerted mechanism, where the protonation, departure of water, and deprotonation occur simultaneously.
d. The reaction involves a two-step mechanism, but no carbocation intermediate is formed, and the product is 1-butene.
a. The E1 mechanism starts with the protonation of the hydroxyl group by acid, converting it into a better leaving group (water). Water departs, forming a carbocation, followed by the removal of a proton from a beta-carbon by a base, leading to the formation of 2-butene.
For the acid-catalyzed E1 reaction of 2-butanol, which of the following statements are true?
a. The mechanism involves protonation of a hydroxyl group.
b. The mechanism involves formation of an enol.
c. The mechanism involves formation of a 2° (secondary) carbocation.
a. The mechanism involves protonation of a hydroxyl group.
c. The mechanism involves formation of a 2° (secondary) carbocation.
In the reaction to produce adipic acid, each mole of cyclohexane reacts with four moles of H₂O₂. If you added about 3.6 g of H₂O₂ to your reaction mixture (by adding 12 g of a 30% H₂O₂ solution), calculate the mass of H₂O₂ required to fully react with 2.00 g of cyclohexane. How does this relate to atom economy?
Given:
MW of cyclohexane = 82 g/mol
MW of H₂O₂ = 34 g/mol
Molar ratio: 1 mol cyclohexane : 4 mol H₂O₂
a. 1.7 g of H₂O₂ required; the reaction has low atom economy because there is excess reactant.
b. 6.6 g of H₂O₂ required; the reaction has high atom economy because little waste is produced.
c. 3.3 g of H₂O₂ required; the reaction has high atom economy because most of the reactants' mass is incorporated into the final product.
d. 3.3 g of H₂O₂ required; the reaction has low atom economy because a large amount of by-products are formed.
c. 3.3 g of H₂O₂ required; the reaction has high atom economy because most of the reactants' mass is incorporated into the final product.
Which of the following principles of green chemistry are demonstrated in Experiment 6, "Oxidative Cleavage: Synthesis of Adipic Acid"? Select three.
A) Solventless Reaction - Cyclohexene acted as its own solvent, improving atom economy and reducing waste.
B) Use of Hazardous By-products - The reaction produced harmful by-products requiring special disposal.
C) Minimization of By-products - The only by-product formed was water (H₂O), a non-hazardous substance.
D) Recycling of Materials - A product from a previous experiment was reused as a reactant in this experiment.
E) Use of Toxic Solvents - The reaction relied heavily on toxic organic solvents.
A) Solventless Reaction - Cyclohexene acted as its own solvent, improving atom economy and reducing waste.
C) Minimization of By-products - The only by-product formed was water (H₂O), a non-hazardous substance.
D) Recycling of Materials - A product from a previous experiment was reused as a reactant in this experiment.
Each year, 2.5 billion kilograms of adipic acid are produced industrially via nitric acid oxidation. Given that for every mole of adipic acid produced, one mole of nitrous oxide (N₂O) is emitted, approximately how many moles and what mass of N₂O are released into the atmosphere annually?
A) About 1.71 × 10¹⁰ moles of N₂O and 753 million kilograms (7.53 × 10⁸ kg) of N₂O.
B) About 1.71 × 10⁸ moles of N₂O and 75.3 million kilograms of N₂O.
C) About 2.5 × 10¹⁰ moles of N₂O and 1 billion kilograms of N₂O.
D) About 1.46 × 10¹⁰ moles of N₂O and 650 million kilograms of N₂O.
A) About 1.71 × 10¹⁰ moles of N₂O and 753 million kilograms (7.53 × 10⁸ kg) of N₂O.
In a reaction where one mole of cyclohexene reacts with four moles of hydrogen peroxide (H₂O₂) to form adipic acid, you added about 3.6 grams of H₂O₂ to your reaction (using 12 grams of a 30% H₂O₂ solution). Given that cyclohexene has a molar mass of 82.15 g/mol, calculate the mass of H₂O₂ needed to react with 2.00 g of cyclohexene. How does this relate to the principle of atom economy?
A) 3.31 g of H₂O₂ is needed, meaning the reaction uses nearly the exact stoichiometric amount, aligning with the principle of atom economy by minimizing waste.
B) 4.50 g of H₂O₂ is needed, and the reaction uses an excess, violating the principles of atom economy.
C) 2.00 g of H₂O₂ is needed, showing that the reaction is inefficient and does not support atom economy.
D) 5.00 g of H₂O₂ is needed, leading to significant waste and poor atom economy.
A) 3.31 g of H₂O₂ is needed, meaning the reaction uses nearly the exact stoichiometric amount, aligning with the principle of atom economy by minimizing waste.
Which of the following statements best describes the green chemistry advantage of using hydrogen peroxide (H₂O₂) in the oxidation reaction to produce adipic acid?
A) Hydrogen peroxide decomposes into nitrogen gas (N₂), which is less hazardous than traditional waste products.
B) Hydrogen peroxide transfers oxygen atoms and produces only water (H₂O) as a byproduct, minimizing hazardous waste.
C) Hydrogen peroxide reacts to form manganese dioxide (MnO₂), which is easy to dispose of.
D) Hydrogen peroxide acts only as a solvent and does not participate in the reaction.
B) Hydrogen peroxide transfers oxygen atoms and produces only water (H₂O) as a byproduct, minimizing hazardous waste.
Why is it important to reflux the reaction mixture during the oxidation of cyclohexene to adipic acid?
A) To ensure the complete evaporation of cyclohexene into the condenser.
B) To keep hydrogen peroxide from decomposing too rapidly.
C) To prevent cyclohexene from leaving the reaction vessel by condensing it back into the reaction mixture.
D) To cool the reaction mixture below 0°C to stop side reactions.
C) To prevent cyclohexene from leaving the reaction vessel by condensing it back into the reaction mixture.
At the 30-minute and 45-minute marks of the oxidative cleavage reaction reaction, you are instructed to wash down the condenser with hot DI water. What is the primary reason for this step?
A) To ensure that any condensed cyclohexene is returned to the reaction mixture for continued reaction.
B) To remove unreacted hydrogen peroxide.
C) To lower the temperature of the reaction mixture.
D) To clean the condenser for the next experiment.
A) To ensure that any condensed cyclohexene is returned to the reaction mixture for continued reaction.
During the transfer of the reaction mixture while it is still hot, why must you avoid pipetting up any phase transfer catalyst?
A) It can react violently with water.
B) It can contaminate the product and affect purity.
C) It is highly toxic and must remain in the reaction flask.
D) It can cause the reaction to restart unexpectedly.
B) It can contaminate the product and affect purity.
How do you confirm the identity of the adipic acid product at the end of the experiment?
A) By measuring its density and comparing it to that of pure adipic acid.
B) By determining its boiling point and measuring pH.
C) By checking its melting point and recording an IR spectrum.
D) By observing its solubility in cold water.
C) By checking its melting point and recording an IR spectrum.
Miscible mixture pressure equation
P total = xA P°A + xB P°B
Where xA, xB: mole fractions of components A and B
P°A, P°B: Vapor pressure of pure components
P total = Total vapor pressure above the solution
Immiscible mixture pressure equation
P total = P°A + P°B
Where P°A, P°B = Each component exerts its full vapor pressure regardless of the presence of the other
Steam distillation
A separation process used to isolate compounds at temperatures below their decomposition temperatures. It is carried out by bubbling steam through the material and distilling off the immiscible liquids
-Gaseous mixture travels upwards to a condenser which then condenses the vapor to liquid so that it can be collected