15 - Mangetic and Chemical Properties
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Magnetic field strength
measure of strength of magnetic field in free space
independent of a medium
units are amperes per meter (A/m)
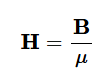
Magnetic Flux density
Included magnetic response of material that field passes through
dependent on medium
units are teslas (T) or weber/m2
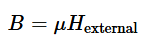
permeability, µ
units are henrys per meter (H/m)
µr is relative permeability
µ0 is the permeability of free space =
4π x 10-7 H/m
corrosion
degredation of a material by chemical reactions with its enviorment
galvanic action
caused when metallic ions differ in oxidation potenial
if metals are similar in oxidation potention corrosion can be slow or negligible
if metals are very dissimilar, corrosion is much faster
anode reaction
metal with higher potential acts as anode and will corrode
cathode reaction
metal with lower potential acts as cathode and is unchanged
fretting corrosion
when metals rub and slide
combination of wear and chemical corrosion
happens on metals that depend on film of surface oxide for protection