A-level Physics OCR A module 6 Particles and Medical Physics
1/147
Earn XP
Description and Tags
All the flash cards from the PMT OCR A A-level Module 6 Particles and Medical Physics module.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
148 Terms
What is a capacitor?
An electrical component that stores charge on 2 separate metallic plates
An insulator called a dielectric is placed between the plates to prevent the charge from travelling across the gap.
What is capacitance
The charge stored per unit potential difference across the two plates. C=Q/V measured in Farads. 1F=1CV-1
What is the relative permittivity/dielectric constant
The ratio of the capacitances of a capacitor with and without the dielectric in place.
ϵr = Q/Q0
Greater the relative permittivity, the greater the capacitance of the capacitor.
What does area under the graph of charge against pd represent?
The energy stored by the capacitor.
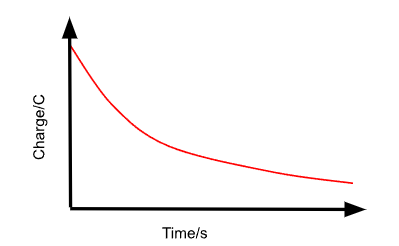
Describe the Q against t graph for the discharging of a capacitor through a resistor.
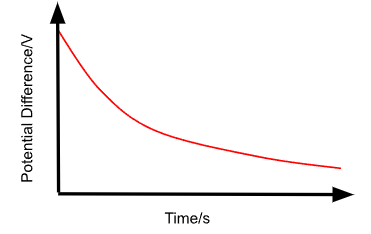
Describe the V against t graph for the discharging of a capacitor through a resistor
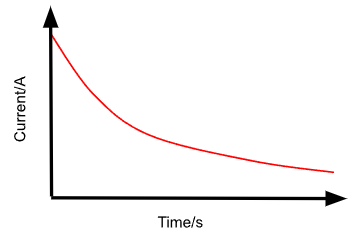
Describe the I against t graph for the discharging of a capacitor through a resistor.
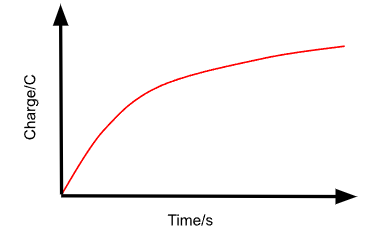
Describe the Q against t graph for the charging of a capacitor through a fixed resistor
What is the time constant (capacitors)
Time it takes for the charge in a capacitor to fall to 37% of its initial value
How was 37% derived when using the time constant?
Start with Q = Q0e-t/CR
When t = RC the formula becomes Q = Q0e-1
e-1 = 0.37
What is the half time of a capacitor?
T½ = (RC)ln(2)
How does a capacitor charge up?
Electrons move from negative to positive around the circuit
The electrons are deposited on plate A, making it negatively charged
Electrons travel from plate B to the positive terminal of the battery, giving the plate a positive charge
Electrons build up on plate A and an equal amount of electrons are removed from plate B, creating a potential difference across the plates
When the p.d across the plates = source p.d the capacitor is fully charged and current stops flowing.
Describe and explain in terms of the movement of electrons how the p.d across a capacitor changes when it discharges across a resistor.
Electrons move in opposite direction than when the capacitor was charging up
Charge on plate A decreases as it loses electrons, and plate B gains electrons, neutralising them
P.d decreases exponentially across the plates
State uses of capacitors
Flash photography
Nuclear fusion
Backup power supplies
What 2 factors affect the time taken for a capacitor to charge or discharge?
Capacitance as affects the amount of charge that can be stored by the capacitors per unit p.d
Resistance of the circuit as affects how quickly the current flows in the circuit.
Define electric field
A region of space in which charged particles are subject to an electrostatic force.
What shape of field do point charges have?
Radial field
How can you model uniformly charged spheres
As a point charge at the centre of the sphere
What do electric field lines show?
The path a positive test charge would take when placed in an electric field.
Which direction do the electric field lines point?
Positive to negative
How is the strength of an Electric Field represented in a diagram?
By how close together the field lines are
Define electric field strength
Force per unit charge on a positive test charge placed in the field
What is the formula for Electric Field Strength?
Electric Field Strength (E) = Force (F) / Charge (q)
What is Coulomb’s Law?
The force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
What is the formula for the force between two point charges?
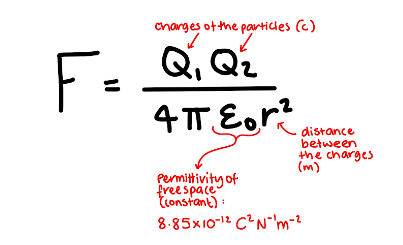
Define Permittivity
The ability of a material to transmit an Electric Field
What is the formula for the Electric Field Strength of a point charge?
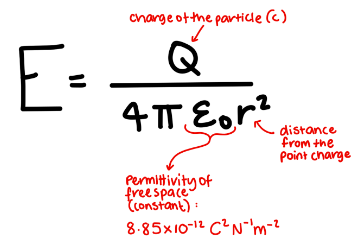
Name similarities between Gravitational and Electric Fields
Both follow inverse square law for the force
Point masses and point charges both produce a radial field.
Field strength defined by force per unit charge/mass
Name some differences between Gravitational and Electric Fields
Gravitational fields are always attractive, Electric Fields can be attractive of repulsive.
What is the formula for the work done when moving a charge in an electric field?
W=Fx
Define potential at a point in a Electric Field
The work done per unit charge in moving a positive test charge from infinity to that point in the Electric Field.
What can the motion of charged particles in an Electric Field be modelled as?
Projectile motion
What does the area under a force distance graph for a point charge represent?
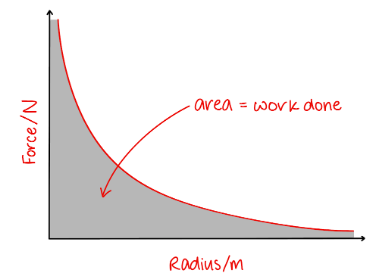
Define Magnetic Field
A region of space in which moving charged particles are subject to a magnetic force.
How can you map field lines around a magnet
place iron filings on a piece of paper and put magnet on paper and filings will align to field.
How do you represent the strength of a Magnetic Field on a diagram?
how close together field lines are
Define Magnetic Flux Density
Force per unit length on a current carrying conductor placed in a Magnetic Field perpendicular to the field lines.
T Tesla
What is the unit of Magnetic Flux Density?
Tesla (T)
1T = 1Nm-1A-1
How do you work out the shape of the field around a current-carrying wire?
Point thumb in direction of conventional current and field goes around in direction of fingers.
Define the motor effect
When a current-carrying conductor is placed within a Magnetic Field it experiences a force perpendicular to the flow of current and the field lines which pushes it out of the field.
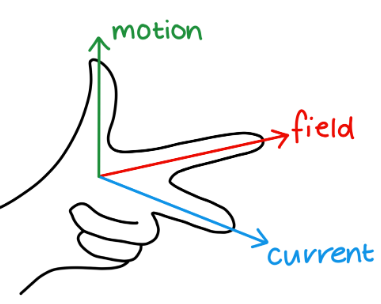
What is Fleming’s left-hand rule:
First finger - Field lines
Second finger - current
Thumb - motion
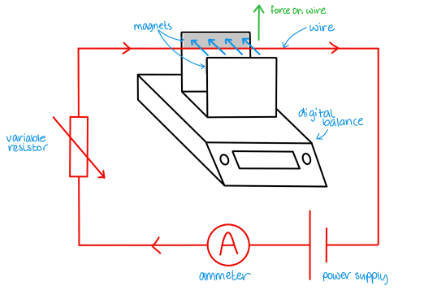
Describe experiment to measure flux density
Place horseshoe magnet on a digital balance and zero it
Connect rigid piece of straight wire to DC supply, variable resistor and ammeter
Align the wire so the force on it acts upwards
Measure the length of the wire in the field
Record extra mass on the balance and use this to calculate the force
Plot a graph of current against mass - gradient gives BL/g
How is F=BQv derived
F=BIL
I=Q/t and L=vt
F=BQvt/t
F=BQv
Why do charged particles move in a circular orbit in a magnetic field?
Force is always perpendicular to the velocity of the partice.
How do you derive the formula for the radius of the circular orbit of a charged particle
mv2/r = BQv
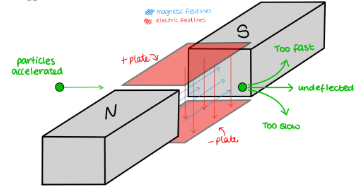
What is the purpose of a velocity selector?
isolate particles of a specific velocity
How does a velocity selector work?
A velocity selector works by applying perpendicular electric and magnetic fields to allow only charged particles with a specific velocity to pass through unaffected.
Define Magnetic Flux
Product of magnetic flux density and the area perpendicular to the field lines
What is the unit for Magnetic Flux?
Weber Wb
1Wb = 1Tm2
Define Magnetic Flux linkage
The product of magnetic flux and the number of turns in the coil
State Lenz’s Law
Induced emf is always in a direction so as to oppose the change that caused it.
State Faraday’s Law
The induced emf in a circuit is proportional to the rate of change of flux linkage throughout the circuit.
How can the peak emf of an A.C generator be increased
Increase the speed of rotation
Increase the magnetic flux density of the field
Increase the cross-sectional area of the coil
Increase the number of turns in the coil
What is the purpose of a transformer?
Change peak value of an alternating PD to a different value. Step up increase it, Step down decrease it.
Describe structure of simple transformer
Two coils, primary and secondary wrapped around two sides of a laminated iron ring. For step up there’s more on the secondary coil.
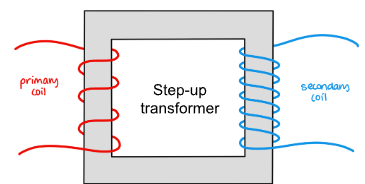
How does a transformer work?
alternating current is run through the primary coil which induces an alternating magnetic field in iron core. this in turn induces an alternating emf in the secondary coil
What role do transformers play in the National Grid?
Step-up increase voltage before the electricity travels long distances to reduce energy lost as heat due to resistance in the wires as electricity passes through them.
How does the alpha-scattering experiment give evidence of a small, dense nucleus?
A few alpha particles bounce back. This wouldn’t happen if the positive charge in the atom was distributed evenly throughout (as in the Plum Pudding Model), which suggests they must be hitting a dense positive charge. The fact it only happens to a very small number of alpha particles shows the nucleus must be small.
How many times bigger is an atom than a nucleus?
Approximately 100,000 times.
What is a nucleon?
A particle that makes up the nucleus: a protons or a neutron.
What is the definition of an isotope?
Isotopes are atoms of an element (with the same number of protons) with a different number of neutrons
What is the strong nuclear force?
The force that holds the nucleus together. It must overcome the electrostatic force of repulsion between protons
Describe the range of the strong force
Repulsive up to 0.5fm. Attractive up to 3fm.
Give a difference and a similarity between particles and antiparticles
Similarity: Mass. Difference: Charge (eg. for protons/anti-protons).
What is a hadron?
A type of particle which is affected by the strong nuclear force.
What are hadrons made of?
Quarks
What are the classes of hadrons?
● Baryon (three quarks)
● Mesons (two quarks)
What are two examples of baryons?
Protons and neutrons.
What are the four fundamental forces?
● Strong nuclear
● Weak nuclear
● Electrostatic
● Gravity
What are leptons?
Fundamental particles which are not subject to the strong nuclear force.
Give some example of leptons
● Electron
● Muon
● Neutrino
● And their corresponding antiparticles
What are the three types of quark?
● Up (u)
● Down (d)
● Strange (s)
State the quark composition of protons and neutrons.
● Proton (uud)
● Neutron (udd)
Give the charges of the up, down and strange quarks (in terms of the electron charge, e).
Up = +⅔e
Down = -⅓e
Strange = -⅓e
What is meant by beta minus decay?
When a neutron turns into a proton, the atom releases an electron and an anti electron neutrino.
Which quark decays in beta minus decay? What does it turn into?
A down quark turns into an up quark.
What quantities must be conserved during the decay of particles?
Charge, mass, baryon and lepton numbers.
What are the defining features of radioactive decay?
Radioactive decay is spontaneous and random - you can’t predict when an individual nucleus will decay
What features of a nucleus might cause it to radioactively decay?
● Too many or too few neutrons
● Too heavy overall (too many nucleons)
● Too much energy
Name 4 types of radiation?
● Alpha
● Beta (plus and minus)
● Gamma
Order Alpha,Gamma and Beta radiation starting with the most ionising?
● Alpha
● Beta
● Gamma
What is an alpha particle?
A particle which contains two protons and two neutrons, the same as a helium nucleus.
How far does a beta particle typically penetrate in air?
50cm - 1m
What materials would be needed to investigate whether a radioactive source was releasing alpha, beta or gamma?
● Alpha - paper
● Beta - ~5mm thick aluminium
● Gamma - thick lead sheet
In beta plus decay, how does the atomic number change?
It decreases.
What is the activity of a source?
The number of radioactive decays per second (measured in Becquerels, Bq).
In the equation A = λN, what do each of the letters/symbols stand for?
A = activity
λ = decay constant
N = number of radioactive nuclei
What is the half-life of an isotope?
The average time taken for the activity of a sample (or the number of radioactive nuclei) to halve.
What isotope is commonly used to find out how old artefacts are?
Carbon-14 (in radiocarbon dating).
What equation is used to convert mass to its energy equivalent?
E = mc2
What occurs when a particle and antiparticle meet?
● Annihilation.
● When a particle and its antiparticle meet, they will annihilate each other and releases two gamma rays.
● Two rays are released in order to conserve momentum.
● The mass of the particles will transform into the energy equivalent.
What is pair production?
When a gamma ray has enough energy to produce a particle and its antiparticle.
What is the mass defect?
The difference between the total mass of all the nucleons separately compared to the mass of the nucleus.
Why is there a mass defect?
Because energy is released as the nucleons bind together into a nucleus.
What is binding energy?
The energy required to separate a nucleus into its constituent parts.
What is nuclear fission?
Where a unstable nucleus splits into 2 smaller nuclei. Often occurs with the larger nuclei.
What is fusion?
When two small nuclei fuse together to create a larger nuclei. The new nucleus has a larger binding energy per nucleon than the old nuclei therefore energy is released in the process.
Which process (fission or fusion) releases the most energy?
Fusion releases a lot more energy per reaction. This is because the change in binding energy is very drastic.
Why is it difficult to make fusion occur on earth?
There is a large repulsion between the two positively charged nuclei, therefore a lot of energy is required to overcome the repulsion and fuse them together. It is hard to get a material that can withstand the heat and be cost effective.
How is fission used in nuclear reactors?
Rods of uranium-235 absorb neutrons and become unstable and then split into two daughter nuclei. It also releases 2 or 3 more neutrons. These then go on to be reabsorbed by another uranium-235.