Types of Reactions in Organic Chemistry - Chp 23
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
63 Terms
Mechanism of the monochlorination of methane
Substitution Reaction


Mechanism of the monochlorination of ethane
Substitution reaction
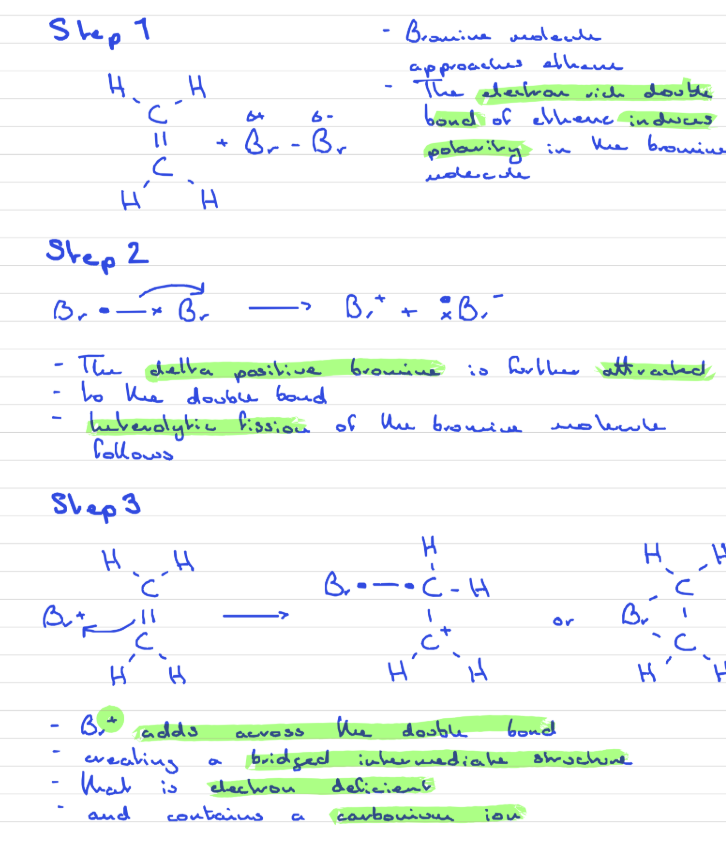
Addition reaction of Ethene with Bromine


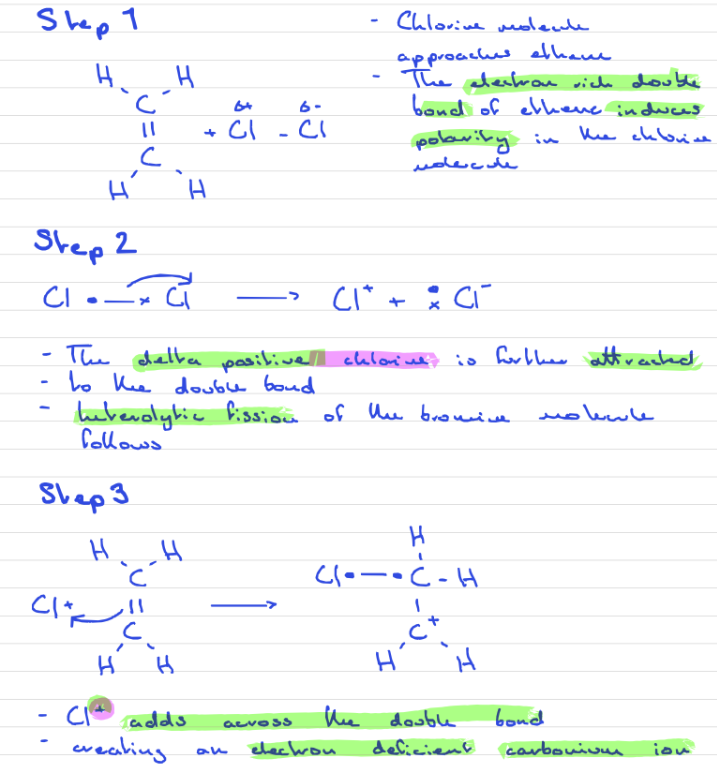
Addition reaction of Ethene with Chlorine


Addition reaction of Ethene with Hydrogen chloride

Synthesis of PVC

Should be chlorines in the PVC structure
Subsitution reaction
a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms
Mechanism
of a reaction is the detailes step-by-step description of how the overall reaction occurs
Chain reaction
is a reaction that continues on and on because a product from one step of the reaction is a reactant for another step of the reaction
Addition reaction
one in which two or more molecules react together to form a single molecule
Polymers
long chain molecules made by joining together many small molecules
Repeating
unit of a polymer is that part of the polymer whose repetition produces the complete polymer chain except for the end groups
Elimination Reaction
is one in which a small molecule is removed from a larger molecule to leave a double bond in the larger molecule
Redox reaction
occurs whenever there is a transfer of electrons from one chemical species to another
Give an example of a non-flammable organic compound:
a fully halogenated alkane
such as bromochlorodifluoromethane
Why are fully halogenated alkanes no longer being used?
negative impact on the environment
Name 2 common oxidising agents:
Potassium permanganate (VII) - KMnO4
Sodium dichromate (VI) - Na2Cr2O7
- when acidified using conc. sulfuric acid they are strong oxidising agents, and are themselves reduced
Describe the colour of acidified KMnO4 in its different oxidation states:
Purple - contains the MnO4- ion (Mn - ON of +7)
Brown - intermediate oxidation state (Mn - ON of +4)
Colourless - final oxidation state (Mn - ON of +2)
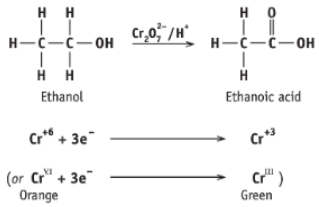
Describe the colour of acidified Na2Cr2O7 in its different oxidation states:
Orange - contains the Cr2O7-2 ion (Cr - ON of +6)
Green - final oxidation state (Cr - ON of +3)
What are primary alcohols oxidised to?
Primary alcohol → Aldehyde → Carboxylic Acid
What are secondary alcohols oxidised to?
Secondary Alcohol → Ketones
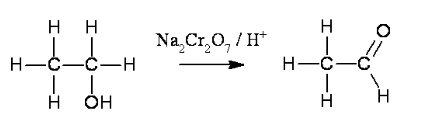
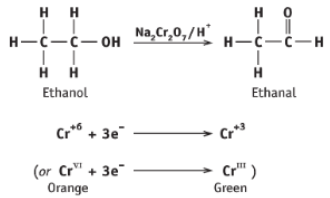
Write the reaction of ethanol with acidified sodium dichromate (VII)
Describe the reaction for the preparation of ethanal:
Set up apparatus for distillation.
Place water and anti bumping granules in the pear shaped flask.
Whilst keeping the pear shaped flask under cold water add conc. H2SO4
Into the dropping funnel add sodium dichromate, water and ethanol. (Solution will be orange.)
Heat the acid until it is just boiling and then remove heat source (reaction is exothermic so temperature will be maintained).
Slowly add the dichromate/ethanol mixture at a rate that keeps the acid bubbling.
Distil as the ethanal is formed, keeping the distillate in an ice bath since ethanal is volatile.
To purify redistill the distillate, collecting the fraction that distils across at 20-23 oC.
What is the limiting reagent in the experiment for the preparation of ethanal?
the oxidising agent is the limiting reagent
the alcohol is in excess
Write the half equations for the oxidation of ethanol to ethanal
Draw the oxidation of ethanol to a carboxylic acid
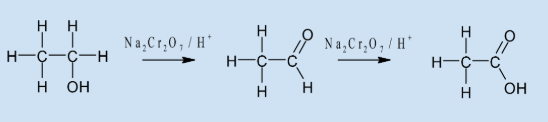
Describe the reaction for the preparation of ethanoic acid
Set up apparatus for reflux
Add sodium dichromate and dilute sulfuric acid into the round bottomed flask. Add some conc. H2SO4 whilst keeping the flask cold.
Place some ethanol and water in the dropping funnel and slowly add the entire contents to the round bottomed flask.
Remove the dropping funnel and when the reaction has subsided reflux the mixture for 20 – 30 minutes using a water bath.
Rearrange for distillation (without a water bath, direct heating required due to temperature rising above 100 oC)
The bold are precautions to prevent excessive heat production
Write the half equations for the oxidation of ethanol to ethanoic acid
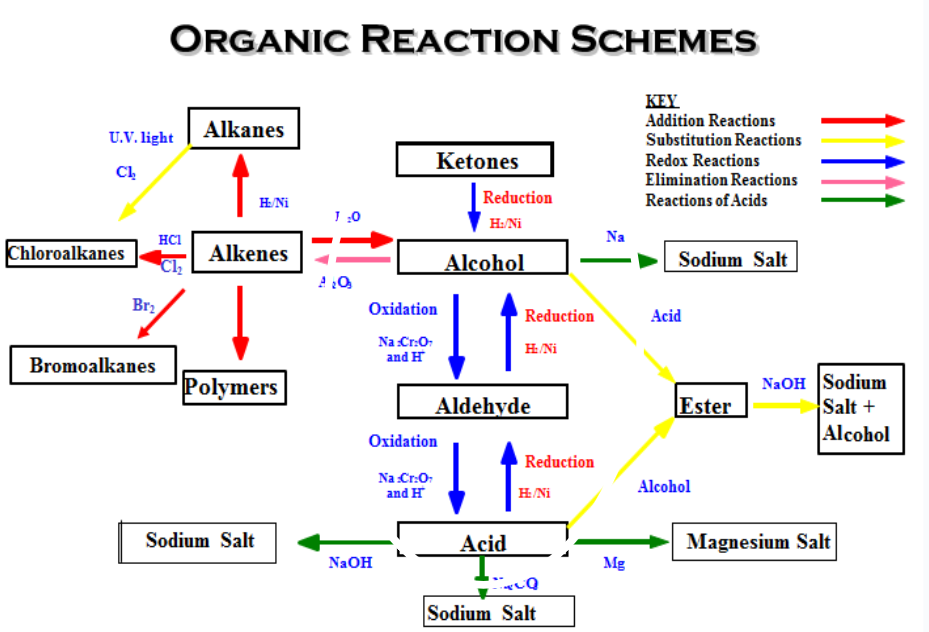
Organic Reaction Scheme
What is esterification?
formation of an ester and water from the reaction between an alcohol and carboxylic acid
What is hydrolysis?
the formation of an alcohol and carboxylic acid from an ester and water
Why can alcohols behave as acids?
the polarity in the O-H bond makes it easier for the hydrogen atom to break off as a H+ ion
Describe how alcohols behave as an acid?
they are very weak acids, 100 times weaker than water
they will react with very reactive metals, such as the alkali metals
they will NOT react with bases such as carbonates or hydroxides
Describe what happen when sodium is added to ethanol?
hydrogen gas is generated
Write an equation for the reaction between ethanol and sodium:

Describe what happens when ethanol is added to some sodium carbonate/hydroxide?
nothing, as ethanol is too weak and acid to react
What are the conditions necessary for a carboxylic acid to be reduced to the corresponding aldehyde and alcohol?
in the presence of hydrogen (reactant) and nickel (catalyst)
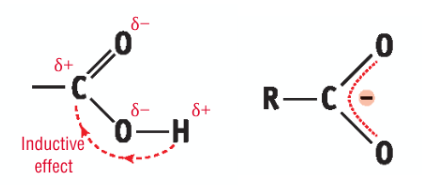
Why can carboxylic acids behave as acids?
Inductive effect - the slightly positive carbon atom in the COH part of the molecule, draws electrons away from the hydrogen atom, so the hydrgoen of the hydroxyl group is lost readily
Stability of the carboxylate ion - when the carboxyl group loses a proton it forms the negative carboxylate ion, forming a resonance hybrid structure where the negative charge is spread over three atoms giving it extra stability, thus allowing it to lose protons readily
Describe the properties of ethanoic acid:
vinegar odour
changes universal indicator paper from green to red, as it is acidic
How can you distinguish between ethanoic acid and ethanol?
add magnesium metal, ethanol will not react with it as it is a weak acid and magnesium is not a reactive enough metal
Paper Chromatography - principle
Difference components of the mixture have different interactions with the mobile phase and stationary phase, so the different components of the mixture will travel different distances along the paper, separating the components of the mixture
Paper Chromatography - processes
add solvent to a chromatography tank
apply spot of mixture to the chromatography paper
dry
place in chromatography tank so that sport is just above solvent
components of mixture separate out as the mobile phase moves up through the paper
Paper Chromatography - use
separate a mixture of colours/dyes
Gas Chromatography - principle
Different components of the mixture have different interactions with the stationary phase and mobile phase, thus the different components will travel at different speeds along the column separating the components of the mixture
stationary phase - liquid supported on a porous bed inside a long coiled column
mobile phase - inert gas
Gas Chromatography - processes
injection
transport of the sample along the column
separation in the column
detection
Gas Chromatography - use
measure the level of alcohol in blood
carry out drug tests on athletes
Mass Spectrometry
GC-MS Chromatography + applications
is a method that combines the features of GL chromatography and mass spectrometry to identrify different substances within a test sample
applications
drug detection
environmental analysis
identification of unknown samples
High Performance Liquid Chromatorgaphy (HPLC) - principle
different components of the mixture have different tendencies to absorb onto very fine particles of a solid in the HPLC column and solvent that is pumped under pressure through columns so the different components will travel different speeds along the column thus separating the components of mixture
High Performance Liquid Chromatography - processes
injection
transport of the sample along the column
separation in the column
detection
High Performance Liquid Chromatography - use
identify the presence of growth promoters in meat/vitamins in food
identify the presence of drugs
Infra Red Absorption Spectrometry - principle
molecules of a substance absorbs infra red light of different frequencies, and does so by vibrations of the bonds in the molecules, the combination of frequencies absorbed is particular to the molecules of that substance
Infra Red Absorption Spectrometry - processes
infra red radiation passes through the sample
the sample absorbs IR radiation at specific wavelengths which are detected
absorption spectrum is produced
Infra Red Absorption Spectrometry - use
qualitative determination of compounds in plastics or drugs
identifies compounds, but not the concentration
Ultraviolet Absorption Spectrometry - principle
absorption of ultraviolet radiation by molecules results in the promotion of electrons from their ground state energy levels to higher energy levels, and the absorbance is directly proportional to concentration
Ultraviolet Absorption Spectrometry - process
ultraviolet light is passed through the sample and a blank
the sample absorbs ultraviolet radation at a specific wavelengths which are detected
absorption spectrum is produced
Ultraviolet Absorption Spectrometry - use
quantitative determination of organic compounds such as drug metabolites/plant pigments
gives concentration, but cannot identify usually
Atomic Absorption spectrometry - principle
atoms in the ground state absorb light of a particular wavelength characteristic of the element, and absorbance is directly proportional to concentration
Atomic Absorption spectrometry - process
sample solution is sprayed into the flame and the sample element is converted into atoms in the element
ground state atoms absorb radiation from a source made from the element
absorbance is measured
Atomic Absorption spectrometry - use
detection and measuring concentration of heavy metals
detection and measuring concentration of water pollutants
Colorimetry - principle
if a solution is coloured then the intensity of the colour is proportional to the concentration
the percentage of light absorbed by the coloured solution in the colorimeter is proportional to the concentration
Colorimetry - process
light of a particular wavelength is passed through a number of samples of known concentration
a graph of absorbance against concentration is plotted
the absorbance of the unknown is noted and using the graph the concentration of the unknown can be found
Colorimetry - use
determination of the concentration of lead in water/fertilisers
determination of the concentration of free chlorine in pool water