g - Esters
1/5
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
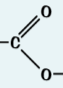
Esters functional group
-COO
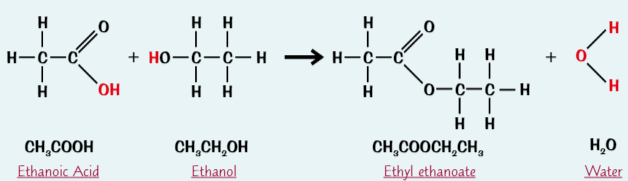
Esters are formed from…
an alcohol + carboxylic acid
in presence of acid catalyst (e.g. conc. sulfuric acid)

Ethyl ethanoate
Ester produced when ethanol + ethanoic acid react in presence of acid catalyst
Naming esters
Names end in ‘-oate’
First part of name - alcohol
Second part of name - acid

Esters are used…
in perfumes as many of them have pleasant smells + volatile (evaporate easily)
Also used to make food flavourings e.g. esters that taste of apple, orange etc.
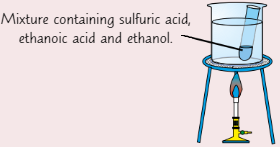
Preparing sample of ester
How to make ethyl ethanoate:
Add a few drops of conc. sulfuric acid to boiling tube using dropping pipette
Add 10 drops of ethanoic acid
Add equal volume of ethanol
Place boiling tube in beaker of water + place on tripod
Heat using Bunsen burner until water boils, then turn off Bunsen
After 1 min, remove tube and allow to cool
Once cool, pour mixture into test tube of sodium carbonate solution + mix
Layer of ester should form on top of solution