ap chem unit 6 thermodynamics
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
what is the relationship between temperature and kinetic energy and velocity? what about mass and velocity?
as temperature increases, KE and velocity increases
as mass increases, velocity decreases
what happens when two systems at different temperatures come into contact (particles collide)?
the hotter one transfers energy (heat) to the cooler one until the temperatures are equal. at this point, the average KE of the particles of each system becomes the same as well. when this is accomplished and there is no further transfer of heat, thermal equilibrium is accomplished
calorimetry
the experimental technique used to measure energy changes in a chemical system
the amount of energy required to raise 1g of a substance by 1 °C
what is specific heat capacity?
q=mc∆T
q: amount of energy transferred (J)
m: mass (g)
c: specific heat capacity (J/g°C)
∆T: change in temperature (Tf - Ti)
no need to convert between K and C but you do need to convert if mass is not g and if q is not J
how do you calculate the amount of energy transferred?
if the temperature of the heat bath (surroundings) goes up, the reaction (system) must have released energy and is exothermic
-qsystem=qsurroundings
system loses E (negative q) while surroundings gain E (positive q)
how do you know if a reaction is exothermic when measuring the temperature?
if the temperature of the heat bath (surroundings) goes down, the reaction (system) must have absorbed energy and is endothermic
qsystem=-qsurroundings
system gains E (positive q) while surroundings lose E (negative q)
how do you know if a reaction is endothermic when measuring the temperature?
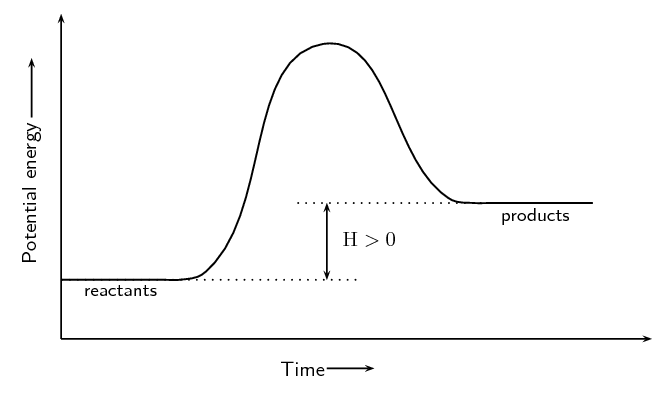
positive ∆H, meaning endothermic
E absorbed
A+B+energy —> C+D
what does the graph of an endothermic reaction look like?
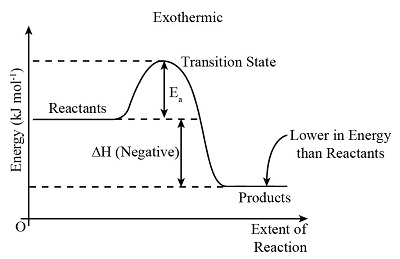
negative ∆H, meaning exothermic
E released
A+B —> C+D+energy
what does the graph of an exothermic reaction look like?
exothermic reactions
whichever reaction has greater magnitude of enthalpy tends to be more spontaneous
example: -250 kJ more spontaneous than -75 kJ
which reactions, endothermic or exothermic, tend to be more spontaneous?
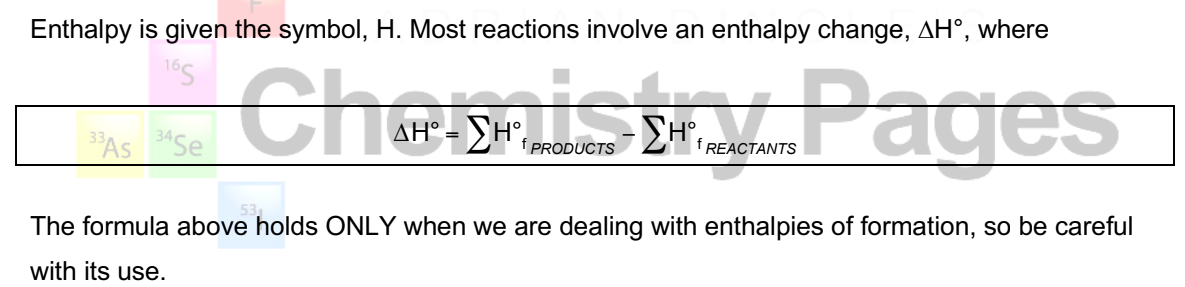
how do you calculate enthalpy (∆H) using enthalpies of formation?
T of the solid increases at a constant rate until it begins to melt (reaches its MP)
using q=mc∆T here
when melting begins, the temperature is constant until the soli has all turned to liquid
using q=∆Hfus · mol
∆Hfus : heat of fusion; used for melting/freezing (E required to change 1 mol of water to ice or vice versa; kJ/mol)
T of the liquid increases at a constant rate until it begins to boil (reaches its bp)
using q=mc∆T
when boiling begins, the temperature is constant until the liquid has all turned to gas
using q=∆Hvap · mol
∆Hvap : heat of vaporization; used for boiling/condensing (kJ/mol)
T of the gas increases at a constant rate
using q=mc∆T
explain the process observed in heating and cooling curves and what calculations to do at each step
heating curves: solid to liquid to gas
endothermic
cooling curves: gas to liquid to solid
exothermic
since it is a decrease in temperature, you will have a negative E so flip the sign when doing calculations using ∆Hfus and ∆Hvap
describe the phase changes in heating and cooling curves and if they’re endothermic or exothermic
no, E is either being used to change the temperature but not the phase, or it is being used to change the phase but not the temperature
plateau in a heating/cooling curve represents a phase change is occurring
can a substance be changing in temperature and phase at the same time?
because the outer electrons of an atom are attracted to the nuclei of another. this attraction makes them increasingly stable, as all bonding is done for the drive for greater stability
why do atoms bond?
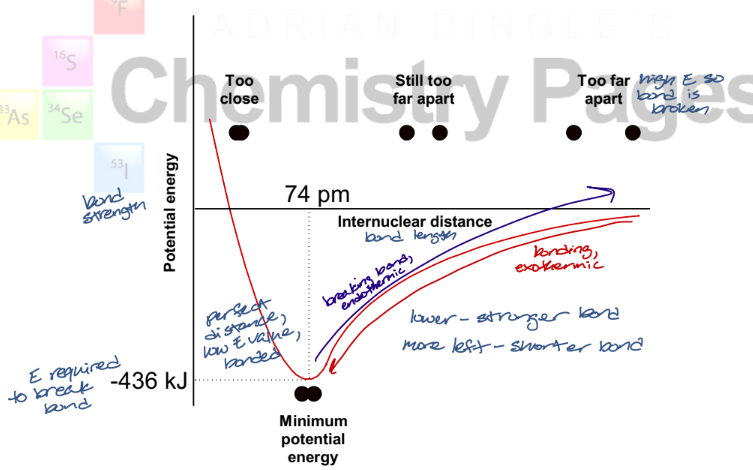
describe what is occurring in this graph
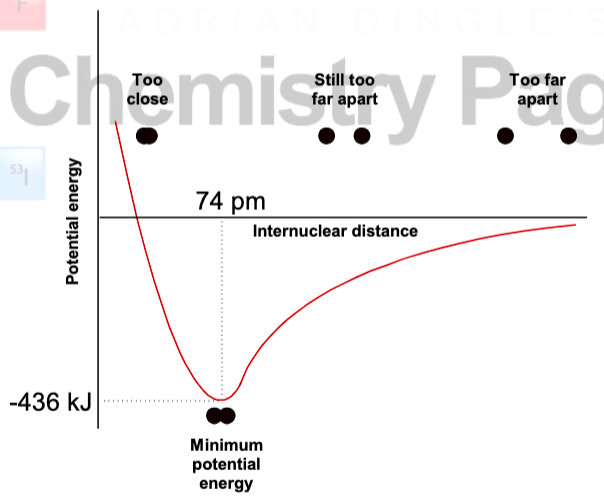
enthalpy of formation is more reliable because bond energies are just averages so they are inherently inaccurate
when calculating the enthalpy change (∆H) for a reaction, are enthalpies of formation or bond energies more reliable?
endothermic: to break a bond, E must be put in
positive energy change
it takes to break
exothermic: when making a bond, E is released
negative energy change
it frees to form
describe the difference between endothermic and exothermic reactions
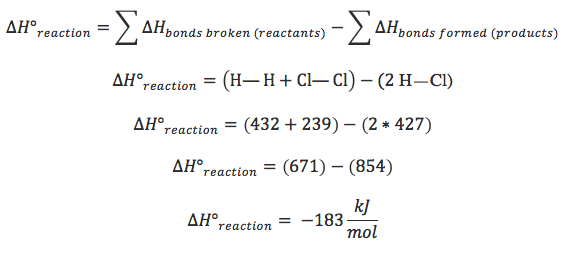
∆H = sum of ∆H of reactants - sum of ∆H of products
how do you calculate ∆H from bond energies?
C: four
H: one
O: two
N: three
S: two
halogens: one
how many bonds do C, H, O, N, S, and the halogens make in organic compounds in bond energy problems?
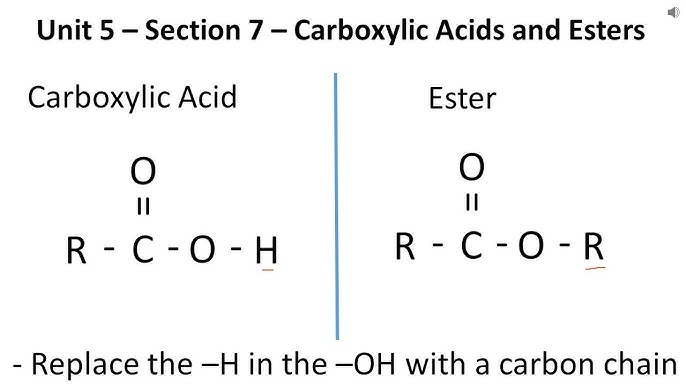
what is the difference between the carboxylic acid group and ester?
how do you calculate a change in enthalpy (∆H°) using enthalpies of formation?
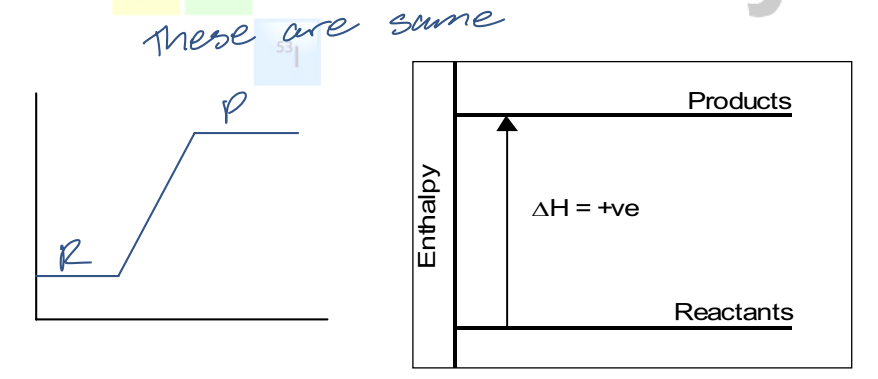
enthalpy of products > enthalpy of reactants
∆H is positive
endothermic reaction
system absorbs E from the surroundings
container will feel cold
what does the enthalpy diagram of an endothermic reaction look like?
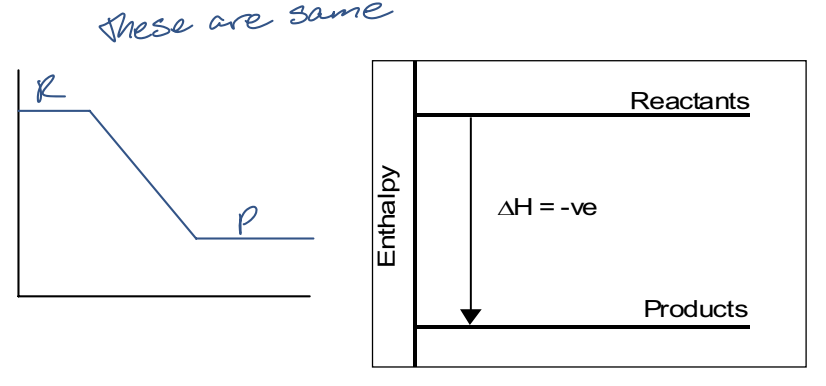
enthalpy of products < enthalpy of reactants
∆H is negative
exothermic reaction
system releases E to the surroundings
container will feel hot
what does the enthalpy diagram of an exothermic reaction look like?
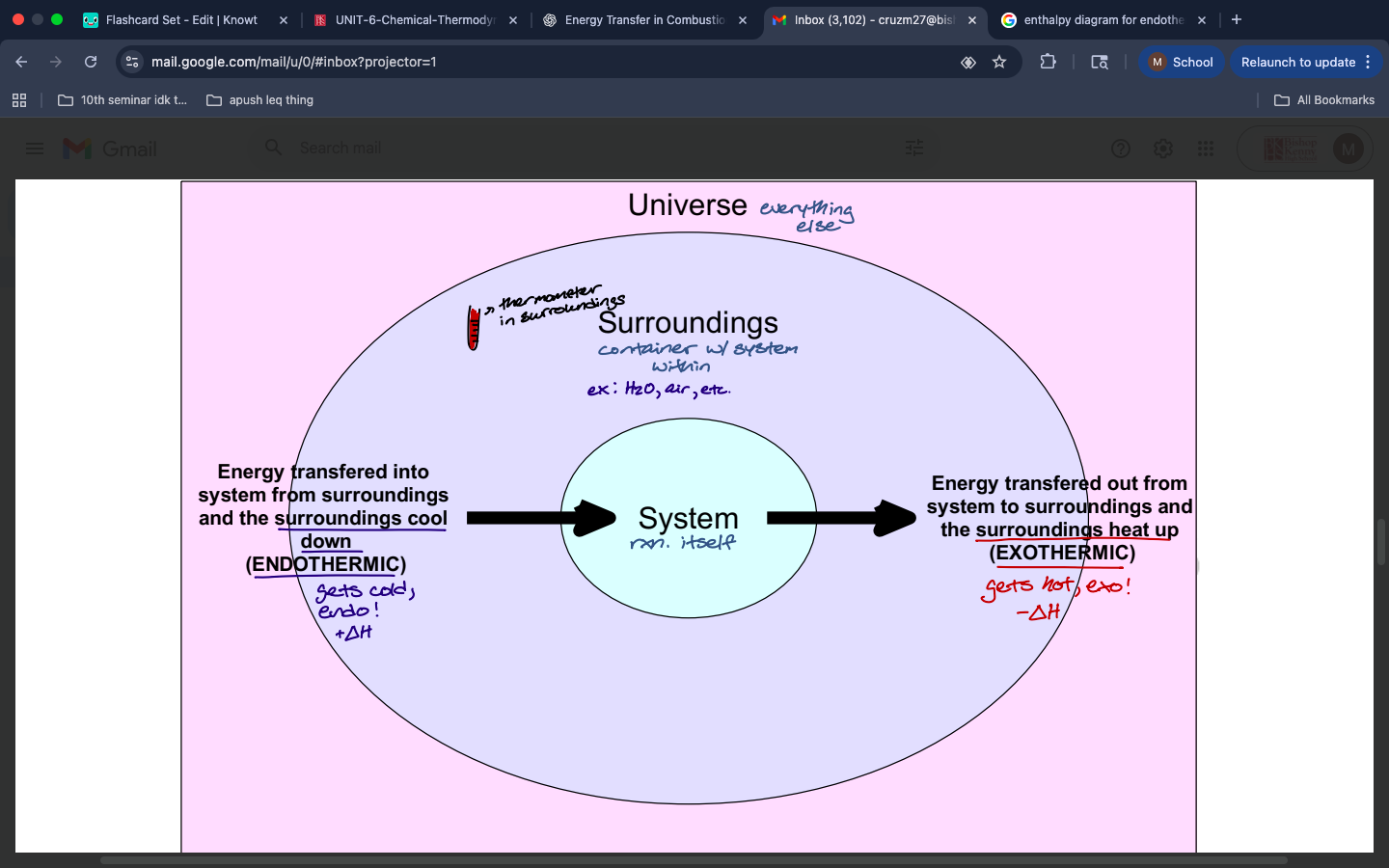
describe the difference between endothermic and exothermic reactions in terms of energy transferring between systems and surroundings
the enthalpy change when one mole of a substance is formed from its elements in their standard states
what is standard enthalpy of formation?
write the full balanced equation the divide each coefficient by the coefficient of the product
how do you write the equation for a standard enthalpy of formation
negative ∆H: exothermic; feels hot; solute-solvent interactions stronger than solute-solute and solvent-solvent
positive ∆H: endothermic; feels cold; solute-solute and solvent-solvent interactions stronger than solute-solvent
when dissolving an ionic substance, what does a negative enthalpy mean? what does a positive enthalpy mean?
the enthalpy change during a reaction depends only on the nature of the reactants and products and is independent of the route taken
what is hess’s law?
∆Hc°
the enthalpy change when one mole of a substance is completely burned in oxygen
what is standard enthalpy of combustion?
write the full balanced equation the divide each coefficient by the coefficient of the reactant being burned in oxygen
reactant + O2(g) —> products
for hydrocarbons, when completely burned in oxygen, only form CO2 and H2O
how do you write the equation for standard enthalpy of combustion?
if the theoretical lattice enthalpy and the experimental lattice enthalpy are close together, it is a good match and the substance is basically completely ionic
if the theoretical lattice enthalpy and the experimental lattice enthalpy are far apart, it is a poor match and the substance is not completely ionic
differences caused by polarization where the ionic substance has some covalent character
how can you tell if a substance is completely or almost completely ionic?
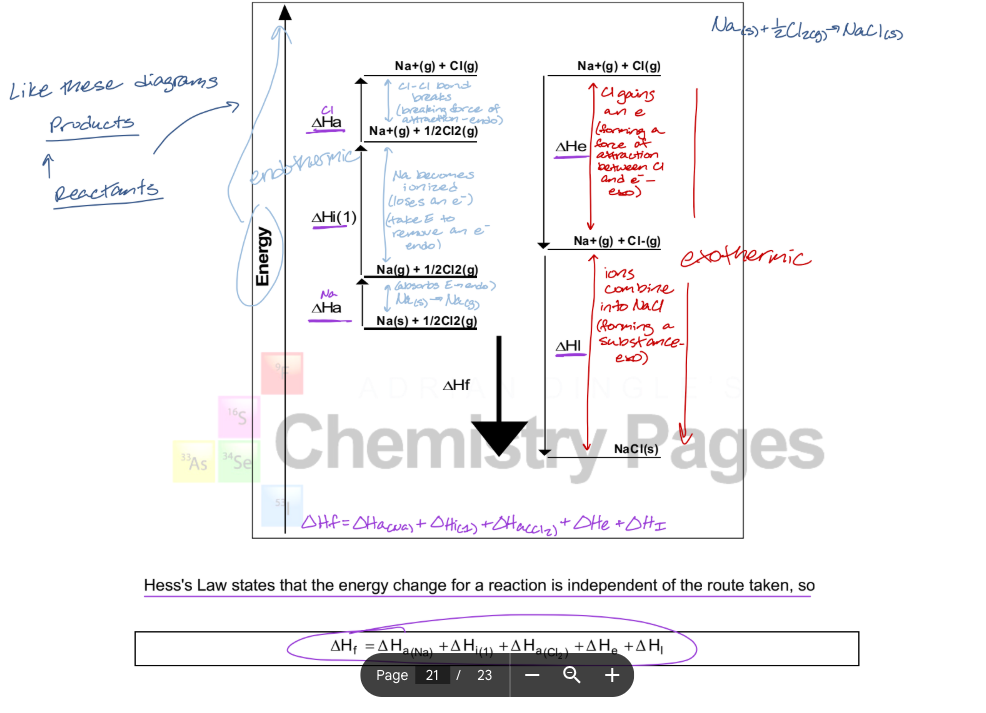
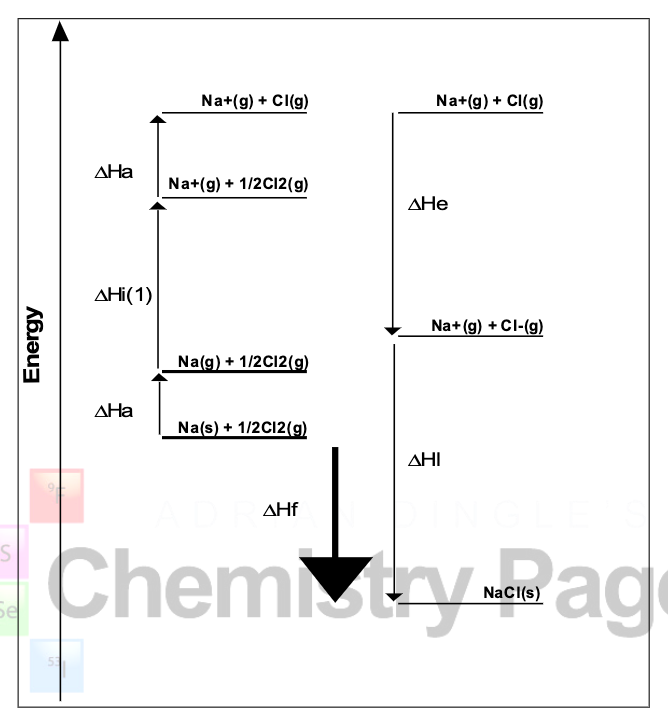
y-axis is energy
processes with arrow pointing up must be endothermic b/c E of system is increasing
processes with arrow pointing down must be exothermic b/c E of system is decreasing
step 1: Na(s) turns into Na(g) — Na absorbs E, so it is an endothermic process
step 2: Na loses an e- — attraction between e- and nucleus takes E to break (endothermic)
step 3: Cl-Cl bond breaks — breaking a force of attraction is endothermic
step 4: Cl gains an e- — forming a force of attraction between Cl and e- and releasing E (exothermic)
step 5: ions combine into NaCl — forming a substance (exothermic)
describe what is happening in this Born-Haber cycle diagram
the reaction will occur without an outside source of energy
-∆H (exothermic) reactions are usually spontaneous
what does spontaneous mean when talking about reactions?
entropy (S) measures the molecular randomness or disorder
an increase in entropy tends to be a spontaneous reaction
what is entropy?
positional entropy: when going from a solid to liquid to gas, S increases
ex: CO2(s) —> CO2(g) ∆S=+
when a solid dissolves in water, S increases (tight packing of molecules is no longer present)
ex: NaCl(s) —> Na+(aq) + Cl-(aq) ∆S=+
during a reaction, when a production of more moles of gas occurs, S increases
ex: 2NH3(g) —> N2(g) + 3H2(g) ∆S=+ because there were originally 2 moles of gas but then there’s 4
all leads to +∆S
when does S increase?
entropy during a process is said to be increasing when S is positive
usually spontaneous
entropy during a process is said to be decreasing when S is negative
when do we say entropy is increasing or decreasing?
∆S° = ∑S°prod - ∑S°react
units: J/K (Joules/Kelvin)
no negative values
you can have a -∆S, but individual S values will be positive
free elements are NOT 0
how do you calculate the change in entropy?
Gibbs free energy (G) is the “free energy” given off and available to do work
sign determines whether reaction is spontaneous
sign of G must be negative for the reaction to be spontaneous
assumed reaction occurs at constant temperature and pressure
what is Gibbs free energy?
-∆G: spontaneous reaction
+∆G: non-spontaneous reaction
using Gibbs free energy, how do you know if a reaction is spontaneous or not?
∆G° = ∆H° - T ∆S°
∆G°: change in Gibbs free energy
∆H°: change in enthalpy
T: temperature in K
∆S°: change in entropy
which reaction is better for calculating Gibbs free energy?
when ∆H is negative and ∆S is positive, it is usually spontaneous
when ∆G is negative, it is always spontaneous
how do you know if a reaction is spontaneous?