Fuel Cells
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
What are Fuel cells
A electrical cell that are supplied by an external source of fuel (eg hydrogen) and oxygen or air. The fuel is oxidised electrochemically within the fuel cell to produce a potential difference.
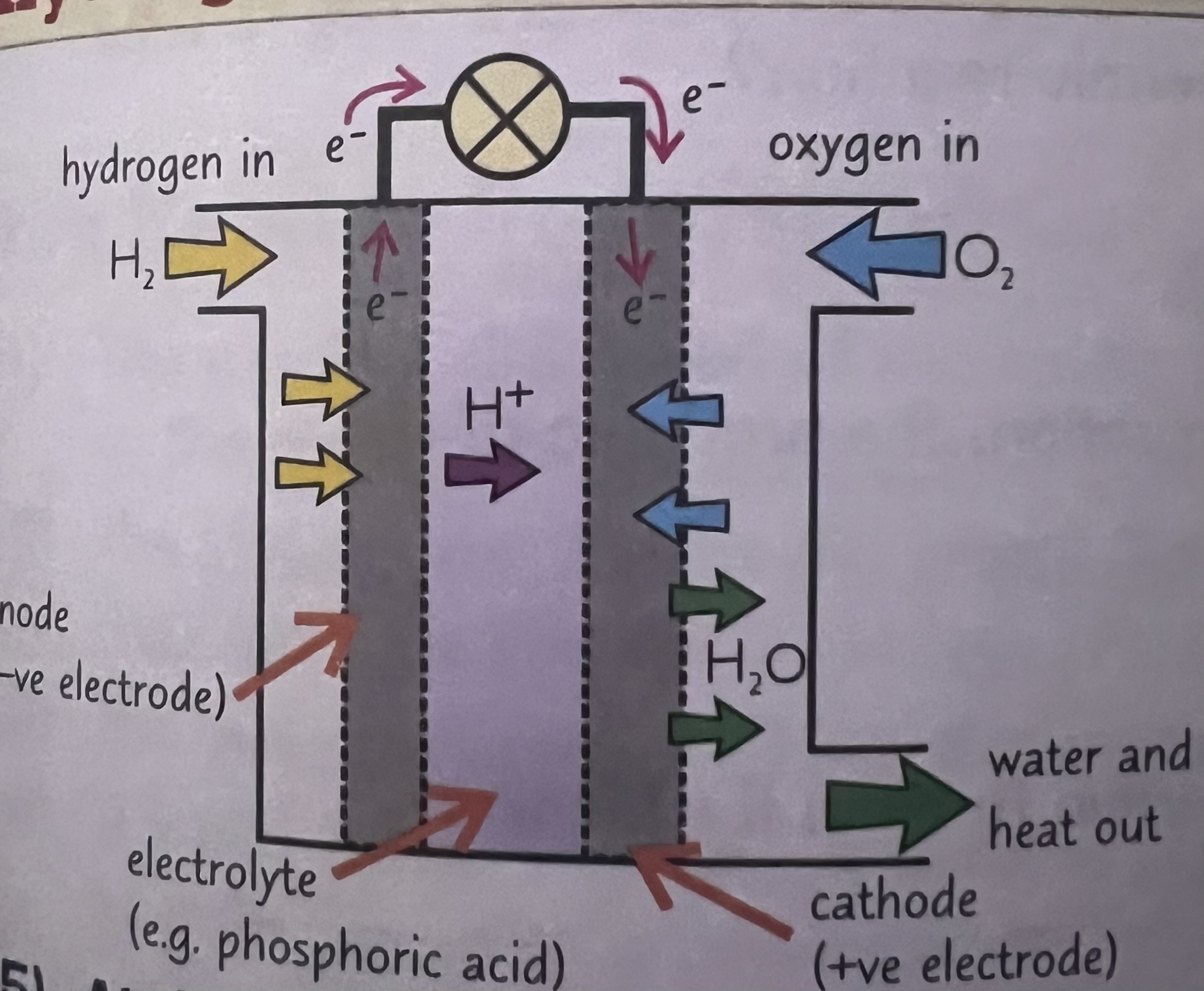
What does a fuel cell produces when it combines with hydrogen and oxygen
Clean water and releases energy
What is the half equation at negative electrode
2H2» 2H+ + 2e-
What’s the half equation at positive electrode
O2 + 4H+ + 4e→2H2O
What’s the overall equation
2H2 + O2» 2H2O
What are the advantages of fuel cells compared to disadvantages of rechargeable batteries
-Fuel cells will produce electricity as long as hydrogen is provided WHILE in rechargeable batteries they eventually run out and need to be recharged
-Fuel Cells do not get less effective the longer they run WHILE rechargeable batteries store less electricity the more charging cycles they go through and eventually needs to be replaced
-Fuel cells are cheaper compared to rechargeable batteries that are more expensive
What are the disadvantages of fuels cells compared to advantages of rechargeable batteries
-Fuel cells run on hydrogen which is an explosive gas and is difficult to store safely WHILE rechargeable batteries have no dangerous fuels required. Although some batteries can catch fire if not manufactured properly
-Fuel cells produce a relatively low potential difference or voltage so several are needed WHILE rechargeable batteries can produce a greater potential difference ever than fuel cells
-Hydrogen is a gas so takes up loads more space to store than a rechargeable battery