Ultraviolet and Visible Spectroscopy
0.0(0)
Card Sorting
1/17
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
1
New cards
Absorption of electromagnetic radiation in the UV and visible regions of the spectrum results in…
excitation of outer shells
2
New cards
Give some key facts about UV spectroscopy.

3
New cards
What can UV / visible spectroscopy be applied to?
Atoms: Atomic orbitals : s, p, d etc
Molecules: Molecular orbitals: Sigma, pi,
Molecules: Molecular orbitals: Sigma, pi,
4
New cards
Show how the electronic energy levels of a molecule of hydrogen change when goes from normal to excited state .
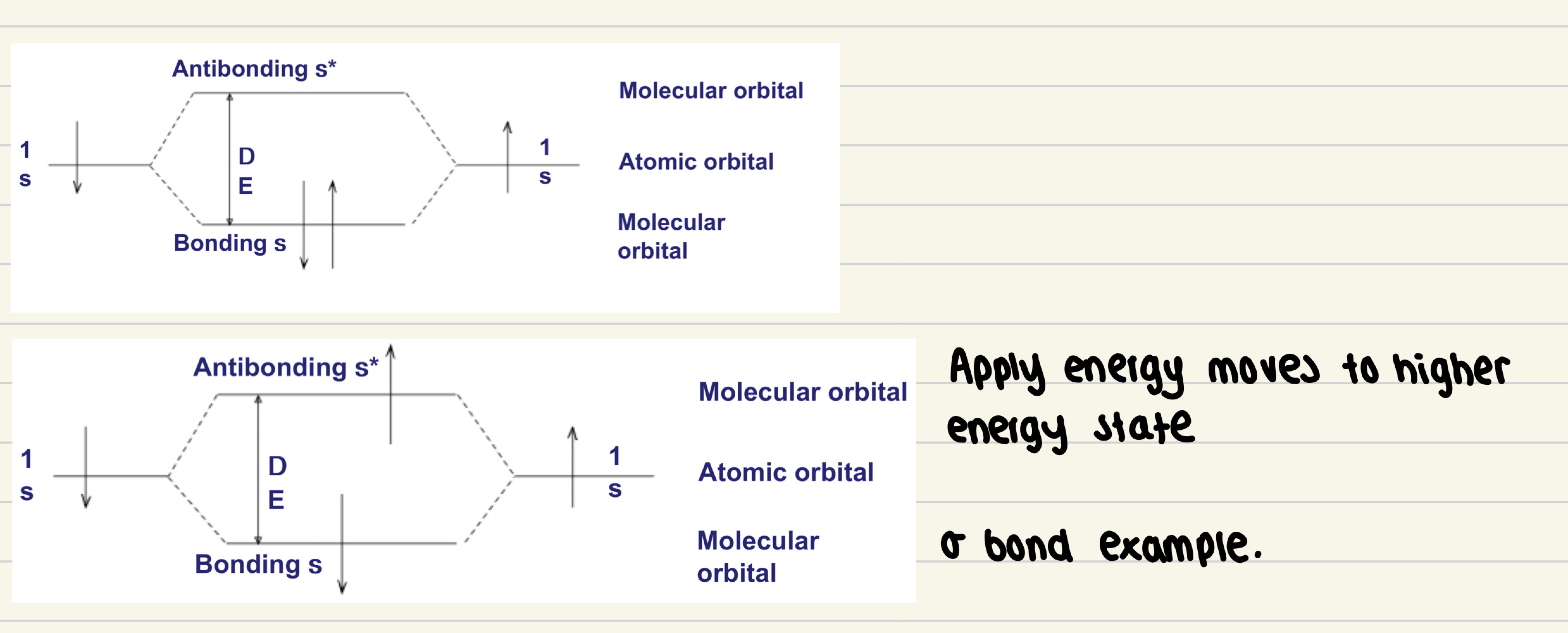
5
New cards
What electron transitions are possible?
Non-highlighted do occur but outside energy range

6
New cards
What are the UV selection rules?
* Simultaneous excitation of more than one electron is forbidden
* An electron must not change its spin during transition
* An electron must not change its spin during transition
7
New cards
Draw a digram to show molecular orbitals which may be involved in electronic transitions.

8
New cards
CHROMOPHORE:
= Is part of a molecule responsible for absorption in the accessible UV or visible region of the spectrum
→ Most of the time will be a %%benzene ring (aromatic)%% or could be %%extended conjugated system%%
→ Most of the time will be a %%benzene ring (aromatic)%% or could be %%extended conjugated system%%
9
New cards
AUXOCHROME:
= is a functional group that has no such absorption of its own but which modifies the absorption properties at a chromophore
10
New cards
What are the characteristics of UV/ visible spectra?

11
New cards
When is fluorescence spectroscopy used?
Will be used in the BP if a molecule is fluorescence
Relevant for biological systems
Relevant for biological systems
12
New cards
What are the characteristics of fluorescent compounds?

13
New cards
Fluorescence depends on…
rigidity of the molecule
14
New cards
Fluorescence differs from absorbance in that…

15
New cards
What are the sources of quenching?

16
New cards
What are the advantages of fluorescence spectroscopy?

17
New cards
What applications of fluorescence spectroscopy?

18
New cards
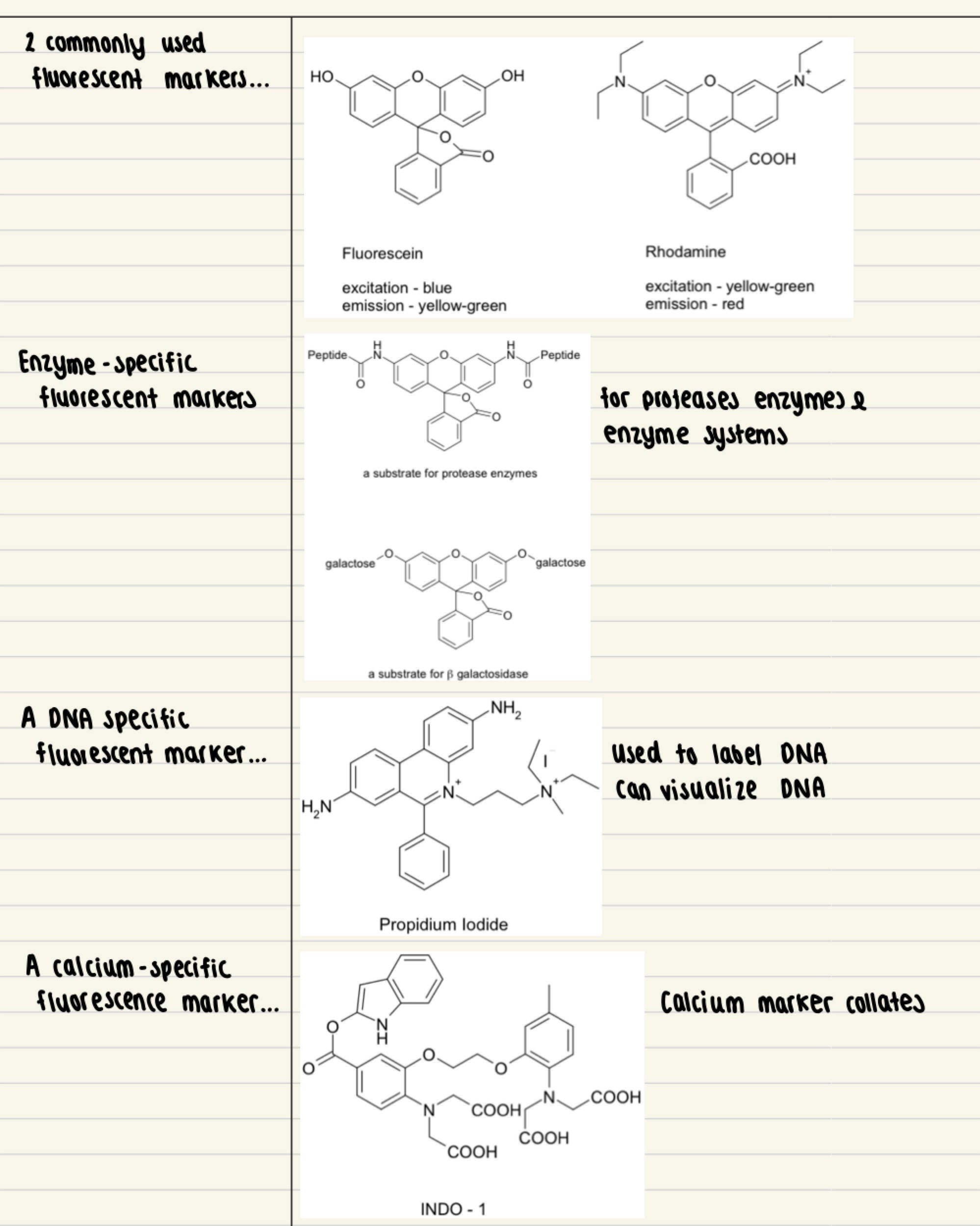
What are the different fluorescent markers you can have?
* Enzyme -specific
* DNA specific
* Calcium specific
* DNA specific
* Calcium specific